Jennifer C. Braimon, Suzanne M. Rieke
Thyroid Disorders
Thyroid disease in its various forms is widely prevalent in the general population. Perhaps 50% of the population have microscopic nodules, 3.5% have occult papillary carcinoma, 15% have palpable goiters, and 10% have abnormal thyroid-stimulating hormone (TSH) levels; 5% of women have overt hypothyroidism or hyperthyroidism.1
Hormones secreted by the thyroid gland influence a variety of metabolic processes in the body. Thyroid function is regulated by TSH, which is secreted by basophilic cells in the anterior pituitary gland in response to the secretion of thyrotropin-releasing hormone (TRH) from the hypothalamus. TRH secretion is regulated in a negative feedback fashion by the thyroid hormones. Low serum levels of thyroid hormones trigger TRH release from the hypothalamus, which in turn causes TSH release from the pituitary. TSH causes increased release of thyroid hormones until a normal serum level is reached. Within the thyroid gland, thyroid function is affected by glandular organic iodine content.
The synthesis of T4 (thyroxine) and T3 (triiodothyronine) requires that adequate quantities of iodine enter the thyroid gland. Iodine enters from the bloodstream and is a constituent of both T4 and T3. These hormones are transported in the bloodstream bound to plasma proteins. The majority of T4 is bound; only a small portion is free. However, it is the free T4 concentration in the serum that indicates thyroidal activity. Approximately 80% of serum T3 is formed in the liver, kidney, and muscle from the deiodination of T4; the remaining 20% is secreted directly by the thyroid.2 Alterations in the regulation of hormone secretion can have varied effects on the body (Box 214-1).
Alterations in the function of the thyroid gland may result in hypersecretion and increased metabolism (hyperthyroidism) or hyposecretion and decreased metabolism (hypothyroidism). Enlargement of the gland may also occur and take the form of localized nodules or generalized goiter. Localized nodules may be benign or malignant and solitary or multiple; goiters may be mild or extensive.
Thyroid Function Testing
Thyroid function can be evaluated in the laboratory through the use of thyroid function tests (TFTs). Thyroid structure and function can be assessed through a variety of imaging techniques and through biopsy.
TSH is the most sensitive indicator of overall thyroid function. Small changes in serum T3 and T4 levels affect TSH secretion in an inverse log-linear relationship. Current techniques allow measurement of serum TSH concentrations as low as 0.01 µIU/ml (third-generation assay, immunometric dual-antibody assay). This is generally the best screening test for thyroid dysfunction. Exceptions include patients with pituitary or hypothalamic (secondary or tertiary) disease and patients immediately after treatment of hypothyroidism or hyperthyroidism (when the TSH response to therapy may lag behind). In addition, various medications and nonthyroidal conditions may affect TSH levels.
TSH measurements are usually sufficient to categorize patients into one of three groups: hyperthyroid (TSH <0.3 µIU/ml), hypothyroid (TSH >4 µIU/ml), and euthyroid (TSH 0.3 to 4 µIU/ml). In a review by Surks and Boucai,3 TSH distributions were found to shift to higher concentrations with age and to vary according to race, with higher concentrations found in whites than in blacks or Hispanics.
Approximately 99% of circulating T4 and T3 is bound to serum proteins. It is the free, unbound T4 that is maintained at a constant level and correlates most with the thyroid state. Free T4 traverses cell membranes to exert its effects on body tissues. Direct measurement by equilibrium dialysis of free T4 is available but is cumbersome and technically demanding and is not for routine use. More commonly, a free thyroxine or a calculated free T4 index that corrects the total T4 (TT4) level for the concentration of thyroxine-binding globulin (TBG) is used to asses the thyroxine level.
TBG determinations are inaccurate in patients with congenital absence of TBG or familial dysalbuminemic hyperthyroxinemia (FDH). Patients with FDH have aberrant albumin that binds T4 (not T3) with increased affinity. In FDH, laboratory tests reveal increased TT4, normal total T3 (TT3), normal TSH, and normal free T4 by equilibrium dialysis. Circumstances that increase TBG include pregnancy, acute hepatitis, and inherited abnormalities and the use of estrogen, oral contraceptives, methadone, or heroin. Decreased TBG results from acromegaly, nephrotic syndrome, cirrhosis, and chronic debilitating disease and from treatment with glucocorticoids, androgens, aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), and some penicillins.
If the TSH level is abnormal, a free thyroxine or free thyroxine index should be obtained. A T3 level should be obtained in the evaluation of hyperthyroidism. T3 determination is useful in diagnosis of T3 toxicosis (normal TT4, decreased TSH, and increased TT3). T3 levels may also be abnormal in what formerly was called euthyroid sick syndrome. T3 levels may be low in patients with nonthyroidal illness.
Autoantibodies to thyroglobulin or thyroid microsomes may be found in patients with autoimmune thyroid disease. Thyroid peroxidase (TPO) is the major microsomal antigen. Anti-TPO antibodies are found in patients with Hashimoto thyroiditis and in less than 85% of patients with Graves disease (autoimmune hyperthyroidism). Antithyroglobulin antibodies are found in 20% of patients with Hashimoto thyroiditis and less than 20% of patients with Graves disease. Up to 15% of individuals in the general population have antibodies to either of these antigens. Quantification of the antibody titers is not clinically useful, although some studies suggest that the severity of thyroid destruction in Hashimoto thyroiditis is proportional to the anti-TPO titer. These tests are particularly useful in the evaluation of patients with atypical manifestations of autoimmune thyroid disease (i.e., isolated ophthalmopathy without signs of hyperthyroidism). They are also predictive of postpartum thyroiditis and neonatal Graves disease.2
Thyroid Imaging
Thyroid scans are used to assess the cause of hyperthyroidism (i.e., Graves disease, toxic nodules, thyroiditis) or the functional status of a nodule, but they are not used to assess thyroid function. Iodine isotope scans (iodine 123 [123I]) are preferred to pertechnetate (technetium Tc 99m, TcO4−) because of the ability of the isotope scan to distinguish between hot and cold nodules. Pertechnetate is concentrated but not bound by thyroid tissues. The scan is performed 20 minutes after the administration of TcO4−. Its advantages include low radiation exposure to the patient, availability, and power of resolution (approximately 5 mm). Rarely, there will be a false-positive result (i.e., “hot” or false uptake) in malignant tissues. Iodine isotopes (123I, 125I, and 131I) are concentrated and bound by thyroid tissues. The scan is performed 4 or 24 hours after the administration of 123I or 125I and 48, 72, or 96 hours after the administration of 131I when it is used to search for metastatic thyroid cancer.
Normally the isotopes are distributed evenly throughout the thyroid gland. Each thyroid lobe is approximately 3 to 4 cm (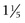 to
to 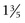 inches) long, 1 to 1.5 cm (
inches) long, 1 to 1.5 cm (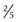 to
to 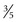 inch) wide, and 1 cm in depth. The isthmus measures about 0.5 cm (
inch) wide, and 1 cm in depth. The isthmus measures about 0.5 cm (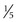 inch) in height and 2 to 3 mm (0.08 to 0.12 inch) in depth. A mottled appearance is seen in Hashimoto thyroiditis or in recently treated Graves disease. An inhomogeneous uptake is also seen in multinodular goiters.
inch) in height and 2 to 3 mm (0.08 to 0.12 inch) in depth. A mottled appearance is seen in Hashimoto thyroiditis or in recently treated Graves disease. An inhomogeneous uptake is also seen in multinodular goiters.
Nodules are classified as hot, warm, or cold according to the concentration of iodine isotope in the nodule in comparison with the rest of the thyroid gland. Hot nodules are usually but not always benign. Many cold nodules (solid or cystic) are benign; however, most malignant neoplasms also appear as cold nodules. The normal radioactive iodine uptake (RAIU) is approximately 30%. Radionuclide imaging cannot be performed for at least 4 weeks in patients who have recently received iodine-containing compounds (i.e., intravenous contrast material). The results may also be inaccurate (falsely low uptake) in patients who are following a high-iodine diet. Exuberant iodine supplementation is becoming more prevalent and may cause a falsely low RAIU. When ordering isotope scans, the health care provider can order RAIU alone or with a scan.
Ultrasonography is used to evaluate the anatomy of the thyroid gland and to differentiate solid from cystic nodules. It is useful in detecting abnormalities larger than 0.5 cm (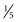 inch) in diameter. It localizes the position and depth of lesions and can be used to guide fine-needle aspiration (FNA). In a study by Papini and colleagues,4 ultrasound features of thyroid nodules predictive of malignant transformation included irregular margins (relative risk (RR), 16.83), intranodular vascular spots (RR, 14.29), and microcalcifications (RR, 4.97). Eighty-seven percent of cancers manifested as a hypoechoic solid nodule on ultrasound.4 Ultrasound cannot be used to visualize substernal goiters because of interference from bone. Computed tomography (CT) and magnetic resonance imaging (MRI) are better suited to assessment of substernal goiters. Cervical lymph nodes are also well visualized on ultrasound. Benign lymph nodes tend to be thin and oval with an echogenic hilum, whereas malignant nodes tend to be round with an undefined hilum and may be vascular. In a retrospective study of the ultrasound appearance of lymph nodes in 63 patients with increased cervical lymphadenopathy, a cystic appearance of cervical lymph nodes was characteristic of metastatic papillary thyroid carcinoma: 70% sensitivity, 100% specificity, 100% positive predictive value, 88% negative predictive value, and 90% accuracy.5,6
inch) in diameter. It localizes the position and depth of lesions and can be used to guide fine-needle aspiration (FNA). In a study by Papini and colleagues,4 ultrasound features of thyroid nodules predictive of malignant transformation included irregular margins (relative risk (RR), 16.83), intranodular vascular spots (RR, 14.29), and microcalcifications (RR, 4.97). Eighty-seven percent of cancers manifested as a hypoechoic solid nodule on ultrasound.4 Ultrasound cannot be used to visualize substernal goiters because of interference from bone. Computed tomography (CT) and magnetic resonance imaging (MRI) are better suited to assessment of substernal goiters. Cervical lymph nodes are also well visualized on ultrasound. Benign lymph nodes tend to be thin and oval with an echogenic hilum, whereas malignant nodes tend to be round with an undefined hilum and may be vascular. In a retrospective study of the ultrasound appearance of lymph nodes in 63 patients with increased cervical lymphadenopathy, a cystic appearance of cervical lymph nodes was characteristic of metastatic papillary thyroid carcinoma: 70% sensitivity, 100% specificity, 100% positive predictive value, 88% negative predictive value, and 90% accuracy.5,6
Fluorine 18 (18F) fluorodeoxyglucose positron emission tomography (18F-FDG-PET) has the highest resolution for detection of aggressive metastatic thyroid cancer lesions. Radiolabeled glucose is injected intravenously, and the scanner produces images that visualize where glucose is used. It identifies differences in how quickly cells metabolize glucose. Cancer cells metabolize glucose more quickly than normal cells do. Nodules with increased uptake on PET scan have a higher risk of malignancy.7
Long-term management of patients with differentiated thyroid cancers includes surveillance for recurrence after initial surgery and radioactive iodine ablation of remnant thyroid tissue. In these patients, disease-free status is defined by negative whole-body scan and undetectable thyroglobulin levels in the absence of interfering antibodies while the patient is receiving thyroid hormone suppression. FDG-PET scanning is especially useful in evaluating patients with differentiated thyroid cancer with elevated thyroglobulin levels and negative 131I whole-body scans for metastases.8 The ability of metastatic thyroid lesions to concentrate 131I is usually indicative of a well-differentiated phenotype.9 Metastases that do not concentrate 131I are typically more aggressive. Most rapidly growing thyroid neoplasms have high metabolic rates. Well-differentiated thyroid tumors were found to retain FDG poorly. FDG volume of more than 125 mL or standard uptake of FDG of more than 10 g/mL suggested a significantly reduced survival. Focal uptake of 18F-FDG can also be seen, however, in inflamed lymph nodes, thyroiditis, and benign thyroid nodules.
Thyroid Cytology
A biopsy specimen is obtained for histologic examination of thyroid tissue (i.e., the architecture is preserved) by closed needle biopsy. FNA biopsy obtains material for cytologic examination only. It is simple and safe but should be performed only by experienced practitioners and is done using ultrasound guidance. Initially, there had been concern about an increased risk of cancer spreading along the needle track from FNA, but this has not been observed. In experienced hands and with ultrasound guidance, FNA biopsy can be very accurate accurate in excluding cancer.
Goiter (Simple, Nontoxic)
Definition and Epidemiology
Enlargement of the thyroid gland is referred to as goiter. It may be caused by hormonal or immunologic stimulation or may result from inflammatory, infiltrative, or metabolic conditions, including iodine deficiency or excess, neoplasia, Graves disease, and thyroiditis.
Nontoxic (simple) goiter occurs when the thyroid gland enlarges in response to inadequate thyroid hormone production. Iodine deficiency remains the most common cause in large areas of Africa, Asia, and South America. The scarcity of iodine in the diet results in the production of TRH, which causes TSH to be secreted in large amounts. The increased TSH has two effects: (1) the retention of all available iodine by the thyroid and (2) the growth of thyroid cells. It is this latter effect that results in thyroid enlargement.
In developed countries, iodine is available in supplemented products such as table salt, fertilizers, animal feeds, and food preservatives. Therefore the most common cause of nontoxic goiter in developed countries is chronic autoimmune thyroiditis.
Pathophysiology
On pathologic examination, simple goiter initially demonstrates a uniformly hypertrophic, hyperplastic, and hypervascular gland. Later, fibrosis may lead to formation of multiple nodules to create a multinodular goiter. These nodules may be “hot” and concentrate iodine or “cold” and not concentrate iodine. When the nodules become autonomous, hyperthyroidism may occur, a condition known as toxic multinodular goiter.
Individuals with a nontoxic goiter may or may not have increased levels of TSH. When levels are normal, it is believed the gland enlarges as a response to impaired hormone synthesis by increasing thyroid mass and cellular activity. In individuals with elevated levels of TSH, the thyroid gland increases mass and activity in response to this stimulation.
Clinical Presentation
Patients with simple goiter usually are seen with either diffuse or multinodular thyroid enlargement. Symptoms such as difficulty swallowing and neck pressure may be present. Undetected and continued growth may result in extension of the thyroid gland downward to a substernal location in the chest. Presentation may include symptoms that result from compression of the trachea, esophagus, and vasculature.
Physical Examination
Examination of the thyroid should begin with observation under a good examining light. The normal gland is rarely visible. It is useful to have the patient extend the neck fully to permit inspection of the gland over the trachea. It is also helpful to observe from the side to identify any enlargement between the cricoid cartilage and the suprasternal notch. Any prominence in this area should be measured with a ruler and recorded; a high likelihood of goiter exists if the prominence is larger than 2 mm (0.08 inch). Having the patient swallow a sip of water enhances visualization and palpation of an enlarged gland. Palpation may be performed either in front of or behind the patient (depending on the practitioner’s comfort), and the texture is noted. The texture of the thyroid can range from extremely soft to relatively firm; the gland may be smooth or may contain palpable nodules. Prominent glands should be measured, and the size recorded. Thyroid size should be categorized as normal or goiter. A small goiter is considered to be one to two times the normal size, and a large goiter is more than twice normal size.
Pemberton sign is used for examination when substernal goiter is suspected. The patient is asked to elevate both arms until they touch the sides of the head. Flushing of the face, cyanosis, and respiratory distress may occur as a result of impingement of structures within the thoracic inlet. Distention of neck veins may also be apparent in these patients.
Differential Diagnosis
Simple goiter must also be differentiated from chronic autoimmune thyroiditis and toxic multinodular goiter. A careful history of symptoms is important. Also, with chronic autoimmune thyroiditis, circulating antimicrosomal antibody levels will be elevated.
Management
![]() Specialist consultation is indicated for large goiters causing compressive symptoms and if indicated consultation with a thyroid surgeon.
Specialist consultation is indicated for large goiters causing compressive symptoms and if indicated consultation with a thyroid surgeon.
The majority of nontoxic goiters grow slowly for many years. The presence of a goiter with no accompanying symptoms or cosmetic concerns is not an indication for treatment. Treatment indications include venous flow obstruction, compression of the trachea or esophagus, progressive enlargement of the entire goiter or individual nodules, neck discomfort, and cosmetic concerns. Treatment options include the following:
1. Surgical treatment, usually bilateral subtotal thyroidectomy, is the preferred treatment in otherwise healthy, young patients, especially in the presence of goiters that grow substernally or continue to enlarge, causing compressive symptoms. There is little evidence that postoperative suppressive T4 treatment prevents goiter recurrence; therefore, it should not be routinely used. Routine postoperative monitoring of TSH may reveal how much functioning thyroid is left and help define those who may need postoperative treatment.10
2. Levothyroxine treatment will suppress TSH, correct any hypothyroidism, and slowly reduce the size of the goiter. However, this therapy may have significant adverse effects, such as decreased bone mineral density, atrial fibrillation, and biochemical hyperthyroidism, if it is not monitored closely. The best candidates for this form of treatment are young patients with small diffuse goiters and a high-normal TSH level. T4 therapy is not recommended for patients with any type of goiter or nodule and a low TSH level because this therapy may cause hyperthyroidism, especially in older adults.10 Some sources recommend a trial of suppressive therapy for patients with a solitary and nonfunctioning nodule, negative fine-needle aspirates, and normal or elevated TSH levels with the goal of keeping TSH at low-normal levels. Such therapy may continue for 6 months to 1 year before reevaluation.
3. Nontoxic multinodular goiter may also be treated with radioiodine to reduce thyroid volume. Therapy with 131I has been found to be an effective alternative with few side effects and is used more commonly in Europe than in the United States.11 This therapy is especially useful in older patients or those with cardiopulmonary disease.
Complications
Nontoxic goiter, even if it is multinodular, has few complications. Of particular concern is the potential for a multinodular goiter to develop autonomous function with ensuing hyperthyroidism. Close monitoring of TSH enables identification of this potential problem. Large goiters, especially those with substernal components, can cause compressive symptoms. Surgical patients with a nontoxic goiter may require special observation for airway maintenance and hormone supplementation if indicated.
Indications for Referral or Hospitalization
Patients who have any indication for treatment of their goiter may require an endocrinology referral for discussion of an appropriate treatment and a surgical referral if thyroidectomy is selected as the treatment of choice.
Life Span Considerations
In general, multinodular goiter increases in frequency with aging. Also, benign diffuse goiter and multinodular goiters do tend to increase in size with age.
Patient and Family Education and Health Promotion
It is important that patients understand the definition and cause of the nontoxic goiter. Patients need to participate in developing the care plan and understand its rationale. Those who live in inland areas or have seafood allergies should use iodized salt. Patients should understand that nontoxic goiter is a manageable, highly livable condition that will not affect their lives in a negative way if it is well controlled.
Thyroid Nodules and Thyroid Cancer
Definition and Epidemiology
A thyroid nodule is a distinct lesion within the thyroid that is radiologically different from the rest of the thyroid. Some palpable lesions do not correspond to radiologic abnormalities. Nonpalpable nodules found on ultrasound or other imaging studies are referred to as incidental thyroid nodules or thyroid incidentalomas. By this definition, thyroid nodules include both solid nodules and cysts.
With ultrasonography, approximately 50% of all single, palpable nodules are found to be in a multinodular gland. In general, nodules larger than 0.5 to 1 cm (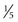 to
to 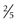 inch) are palpable. Thyroid adenomas are benign neoplastic nodules within a capsule.
inch) are palpable. Thyroid adenomas are benign neoplastic nodules within a capsule.
The prevalence of thyroid nodules depends on the method of evaluation. Palpable thyroid nodules are found in 4% to 7% of the general adult population.12 Autopsy and ultrasound studies have quoted a prevalence as high as 50%. The lifetime risk for development of a thyroid nodule is estimated to be 5% to 10%. Thyroid nodules are common, and only 3% to 5% of all thyroid nodules are malignant.12
Pathophysiology
Thyroid nodules may be caused by adenomas, cysts, carcinomas, multinodular goiters, Hashimoto thyroiditis, and subacute thyroiditis. Less common causes of neck lumps include the effects of prior surgery or 131I, parathyroid cysts or adenomas, thyroglossal cysts, nonthyroidal lesions, and lymphomas.
Thyroid adenomas are benign, monoclonal growths. Benign thyroid tumors include embryonal, fetal, follicular, Hürthle cell, and papillary adenomas. They are distinguished by their characteristic histologic appearance. Malignant thyroid tumors include papillary, follicular, medullary, and anaplastic carcinomas.
Clinical Presentation and Physical Examination
Thyroid nodules are usually asymptomatic and are identified as a lump by patients or by providers during routine thyroid examinations. Increasing numbers of thyroid nodules are being identified incidentally during carotid Doppler ultrasound or other neck imaging studies. Clinical features that increase the likelihood of cancer include history of head and neck irradiation, family history of thyroid cancer, age younger than 20 years or older than 60 years, male gender, and history of multiple endocrine neoplasia type II or medullary thyroid cancer. Familial thyroid tumors also occur in Cowden disease (multiple hamartoma syndrome), Gardner syndrome (development of multiple tumors with autosomal dominant inheritance), and familial polyposis.
An anaplastic tumor may manifest as an enlarging, painful mass associated with hoarseness, dysphonia, dysphagia, or dyspnea. Patients with anaplastic thyroid cancer may have pathologic fractures of the spine or hip or thoracic outlet syndrome. Patients with toxic nodules may show symptoms of hyperthyroidism.
Important features noted during the physical examination include nodule size, consistency, and mobility and the presence and consistency of associated lymphadenopathy. Supraclavicular, anterior cervical, and submandibular lymph nodes should be examined. Although most thyroid cancers feel firm or hard, they can be soft and fluctuant on examination. The presence of a new nodule or enlarging nodule while a patient is receiving T4 therapy is a cause for concern.
Diagnostics and Differential Diagnosis
The initial evaluation of thyroid nodules includes history, physical examination, and measurement of TSH to exclude hyperthyroidism or hypothyroidism. The routine measurement of serum calcitonin (to exclude medullary thyroid cancer) is not useful or cost-effective.13 Elevated TSH levels have been associated with an increased risk of malignant transformation in a thyroid nodule, as well as more advanced stage of differentiated thyroid cancer.14,15
Thyroid ultrasound evaluation should be performed for all patients with a suspected (i.e., incidental) abnormality found on CT, MRI, or 18F-FDG-PET or with known thyroid nodules.12 High-resolution sonography can clearly distinguish between solid and cystic components. The risk of malignancy in 18F-FDG-PET–avid nodules is approximately 33%, and these cancers tend to be more aggressive. Ultrasound characteristics associated with a higher likelihood of malignancy include increased vascular flow to the nodule, hypoechoic nodules, irregular margins, absent halo, microcalcifications, and shape taller than the width in transverse dimension.6 There are also ultrasound appearances that are predictive of benign nodules. Simple cysts are unlikely to be malignant. In a study by Bonavita and coworkers,16 only 1 of 360 malignant nodules demonstrated a spongiform appearance (aggregation of multiple microcystic components making up more than 50% of the nodule volume).
Historically, radionuclide imaging was the first diagnostic test used in the evaluation of solitary thyroid nodules. Although it is true that most malignant thyroid neoplasms appear as cold nodules, most cold nodules are benign. Radionuclide scanning is now used as an initial test if a hyperfunctioning nodule is suspected. It may also be useful in patients with multinodular goiters to target FNA of cold nodules.
According to the revised American Thyroid Association guidelines for patients with thyroid nodules,12 FNA biopsy is the procedure of choice in the evaluation of thyroid nodules. It is safe and technically simple but requires an experienced operator and cytopathologist. False-negative and false-positive rates are less than 5% with experienced users. Cytologic results are sufficient in 85% of biopsies for diagnosis. Ultrasound-guided FNAs have a lower rate of nondiagnostic and false-negative biopsy findings. The revised American Thyroid Association thyroid cancer guidelines (2015) recommend FNA for nodules larger than 1 cm with high-risk history, for solid nodules larger than 1 cm that are hypoechoic, and for complex (solid and cystic components) nodules larger than 1.5 cm with any suspicious ultrasound features.12
FNA cytology result categories include Benign, Follicular Lesion of Undetermined Significance (FLUS), Follicular Neoplasm, Suspicious for Malignancy, Malignancy, and Nondiagnostic. Risks for malignancy in each of theses categories are below 5%, 5% to 15%, 20% to 30%, 50% to 75%, and over 90%. Further evaluation of FNA aspirates for molecular markers may be considered for patients with indeterminate cytology. The use of gene expression classifiers, with high negative predictive value, may help to avoid diagnostic surgery in patients with indeterminate thyroid cytology results.17,18
Management
![]() Specialist consultation with an endocrinologist or interventional radiologist is indicated for ultrasound-guided FNA biopsy of thyroid nodules, and immediate referral to a thyroid surgeon is indicated if compressive symptoms are present.
Specialist consultation with an endocrinologist or interventional radiologist is indicated for ultrasound-guided FNA biopsy of thyroid nodules, and immediate referral to a thyroid surgeon is indicated if compressive symptoms are present.
![]() Immediate emergency department referral is indicated for patients who exhibit respiratory compromise from invasive tumors.
Immediate emergency department referral is indicated for patients who exhibit respiratory compromise from invasive tumors.
Management of thyroid nodule(s) noted after complete history and physical examination and TSH measurement is as follows:
• If TSH is normal, thyroid nodule(s) are evaluated as follows.
• Thyroid ultrasound should be performed on all patients.
• Benign: No further immediate evaluation is necessary. A repeated FNA biopsy should be reserved for enlarging nodules, defined as more than a 50% change in volume or a 20% increase in diameter with at least a 2-mm increase in two or more dimensions. A follow-up ultrasound should be performed in 6 to 12 months. The American Thyroid Association task force strongly recommends against use of T4 suppression.12
• Nondiagnostic: FNA is repeated in 2 to 3 months
• Suspicious for malignancy, or malignancy: Patient is referred to an experienced surgeon. For solitary lesions smaller than 1 cm, lobectomy may be performed. Total thyroidectomy is indicated if there is a history of head or neck irradiation, the tumor extends beyond the capsule, or the lesion is larger than 1 cm. The American Thyroid Association task force recommends against use of radioactive iodine ablation in patients with low-grade thyroid cancer (unifocal cancer <1 cm or multifocal cancer with cumulative size <1 cm) in the absence of higher-risk features. Postoperative radioactive iodine therapy is used to ablate remnant thyroid tissue, to ease early detection of recurrence based on thyroglobulin levels, and to diagnose and treat metastases. Although most studies demonstrate a reduction in recurrence and decreased mortality with remnant ablation, benefits appear to be limited to patients with more advanced stages of thyroid cancer.12,19
Differentiated thyroid cancer is managed as follows:
• Use of the American Joint Committee on Cancer (AJCC) and Union for International Cancer Control (UICC) classification based on TNM (T refers to tumor size, N refers to lymph notes, and M refers to metastases) and age is recommended for all patients with differentiated thyroid cancer because of its usefulness in predicting mortality and its requirement for cancer registries.19
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






