INTRODUCTION
Antithrombotic therapy (i.e., anticoagulants, antiplatelet agents, and fibrinolytics) is used to treat arterial and venous thromboembolic conditions, including acute coronary syndrome, deep venous thrombosis, pulmonary embolism, transient ischemic attack, and ischemic stroke. Moreover, antithrombotic agents help prevent occlusive vascular events in patients at risk for thrombosis due to atherosclerotic arterial disease, atrial fibrillation, medical illness with immobility, or surgical insult. These agents, however, can cause life-threatening complications, primarily serious hemorrhage. Detailed management strategies for thromboembolic disorders are discussed in their respective chapters (see chapter 49, “Acute Coronary Syndromes”; chapter 56, “Venous Thromboembolism”; and chapter 167, “Stroke Syndromes”).
Hemostasis—whether physiologic after accidental injury or pathologic after rupture of an atherosclerotic plaque—is initiated by platelet interaction with the vascular subendothelium and continues with a series of reactions among plasma coagulation proteins that generate the final product of cross-linked fibrin incorporated into the initial platelet plug (see chapter 232, “Tests of Hemostasis”). Arterial thrombi, composed primarily of platelets bound by thin fibrin strands, develop under high-flow conditions, especially at sites of ruptured plaques. Both anticoagulants and platelet-inhibiting drugs may effectively prevent and treat arterial thrombosis. In contrast, venous thrombi form in areas of sluggish blood flow and are composed mainly of red blood cells and large fibrin strands. Anticoagulant drugs are more effective than antiplatelet drugs in preventing venous thromboembolism.
Antithrombotic agents are classified by their mechanism of action. Anticoagulants block the synthesis or activation of clotting factors, interfering with the coagulation cascade at one or more steps. Antiplatelet agents interfere with platelet activation or aggregation. Fibrinolytic agents (often but inaccurately referred to as thrombolytic agents) stimulate the enzymatic dissolution of the fibrin component.
Thrombotic agents are used to diminish bleeding due to either an anithrombotic agent or and acquired or genetic bleeding disorder (see chapters 233 and 235, “Acquired Bleeding Disorders” and “Hemophilias and von Willebrand’s Disease”, respectively).
ORAL ANTICOAGULANTS
Oral anticoagulants are used to (1) stop further thrombosis when the condition already exists (e.g., venous thrombosis), (2) reduce the risk of embolism in patients with thrombotic disease (e.g., venous thrombosis or left ventricular mural thrombus), and (3) prevent thrombi from forming in patients with risk factors for their development (e.g., atrial fibrillation, prolonged immobilization, or prosthetic heart valve) (Table 239-1).
| Clinical Indication | Comments |
|---|---|
| Treatment of Deep Venous Thrombosis and Pulmonary Embolism | |
| Unfractionated heparin 80 units/kg IV bolus followed by 18 units/kg per h continuous IV infusion, with the aPTT checked after 6 h and the infusion adjusted to maintain the aPTT 1.5–2.5 times control with concurrent institution of warfarin | In most cases, heparin and warfarin are started simultaneously, with an overlap of 3–5 d. Warfarin is monitored and dose adjusted to a target INR of 2.0–3.0 in most patients. |
| Enoxaparin 1 milligram/kg SC every 12 h or 1.5 milligrams/kg SC once a day | Monitoring not routinely required |
| Dalteparin 200 units/kg SC once daily or 100 units/kg SC twice daily | Not FDA approved for this indication |
Fondaparinux weight-tiered regimen <50 kg: 5 milligrams SC once a day 50–100 kg: 7.5 milligrams SC once a day >100 kg: 10 milligrams SC once a day | Monitoring not routinely required — — — |
| Rivaroxaban 15 milligrams PO twice daily for 21 d, followed by 20 milligrams PO once daily | Avoid use in patients with CrCl <30 mL/min |
| Dabigatran 150 milligrams PO twice daily (after 5 to 10 d of parenteral anticoagulation) | Avoid in patients with CrCl <30 mL/min or on hemodialysis |
| Apixaban 10 milligrams PO twice daily for 7 d, followed by 5 milligrams twice daily | Reduce dose by 50% if patient taking dual strong VYP3A4 and P-glycoprotein inhibitors |
Streptokinase 250,000 units IV bolus, followed by 100,000 units/h continuous IV infusion for 1–3 d OR Alteplase 100 milligrams IV infused over 2 h OR Tenecteplase weight-tiered single IV bolus <60 kg: 30 milligrams 60–70 kg: 35 milligrams 70–80 kg: 40 milligrams 80–90 kg: 45 milligrams >90 kg: 50 milligrams | Fibrinolytic treatment of deep venous thrombosis and pulmonary embolism is recommended only in carefully selected patients. |
| Prophylaxis of Deep Venous Thrombosis and Pulmonary Embolism | |
| Unfractionated heparin 5000 units SC every 8–12 h | Highest-risk patients for venous thromboembolism should receive every 8 h dosing. |
| Dalteparin 2500 to 5000 IU SC once a day | — |
| Enoxaparin 40 milligrams SC once daily (normal renal function), 30 milligrams SC twice daily (trauma) or 40 milligrams twice daily (obese patients) | — |
| Fondaparinux 2.5 milligrams SC once a day | — |
| Rivaroxaban 10 milligrams PO once a day | — |
| Apixaban 2.5 milligrams PO twice daily | For prophylaxis after hip and knee replacement surgery, initial dose taken 12–24 h after surgery |
| ST-Segment Elevation Myocardial Infarction | |
Aspirin (non–enteric coated) 162–325 milligrams PO once a day Clopidogrel 300 milligrams PO loading dose (consider 600 milligrams if PCI is planned) followed by 75 milligrams PO once a day OR Ticagrelor 180 milligrams PO loading dose, followed by 90 milligrams PO twice daily | All post–myocardial infarction patients should receive aspirin, 162–325 milligrams PO once a day, for an indefinite period (unless contraindicated or if on warfarin). |
Unfractionated heparin 60 units/kg IV bolus (maximum, 4000 units according to the ACC/AHA guidelines or 5000 units according to the ESC guidelines) followed by 12 units/kg per h (maximum, 1000 units) continuous IV infusion adjusted to keep aPTT 1.5–2.5 times control OR Enoxaparin 30 milligrams IV bolus, followed by 1 milligram/kg SC every 12 h for patients <75 years of age or 0.75 milligrams/kg SC every 12 h of patients >75 years of age OR Bivalirudin 0.75 milligram/kg IV bolus followed by 1.75 milligrams/kg per h continuous IV infusion (FDA approved for use in cardiac catheterization laboratory only) | Optimal anticoagulation strategies are not completely defined. |
Streptokinase 1.5 million units IV over 60 min OR Alteplase 15 milligrams IV bolus over 1–2 min followed by 0.75 milligram/kg IV over 30 min (maximum, 50 milligrams) and 0.50 milligram/kg IV over 60 min (maximum, 35 milligrams) OR Reteplase 10 units IV bolus, then a second 10-unit dose at 30 min OR Tenecteplase weight-tiered single IV bolus <60 kg: 30 milligrams 60–70 kg: 35 milligrams 70–80 kg: 40 milligrams 80–90 kg: 45 milligrams >90 kg: 50 milligrams | Early administration is more important than choice of specific fibrinolytic agent. |
| Unstable Angina and Non-ST-Segment Myocardial Infarction | |
| Aspirin (non–enteric coated) 162–325 milligrams PO once a day | Optimal antiplatelet strategies are not completely defined. |
Clopidogrel 300 milligrams PO loading dose (consider 600 milligrams if PCI is planned) followed by 75 milligrams PO once a day OR Prasugrel 60 milligrams loading dose followed by 10 milligrams PO once daily OR Ticagrelor 180 milligrams PO loading dose, followed by 90 milligrams PO twice daily | Dual therapy—aspirin plus another antiplatelet agent—is common. |
Unfractionated heparin 60 units/kg IV bolus (maximum, 4000 units according to the ACC/AHA guidelines or 5000 units according to the ESC guidelines) followed by 12 units/kg per h (maximum, 1000 units/h) continuous IV infusion adjusted to keep aPTT 1.5–2.5 times control Enoxaparin 1 milligram/kg SC every 12 h Glycoprotein IIb/IIIa inhibitor, depending on risk and whether PCI is planned Bivalirudin 0.75 milligram/kg IV bolus followed by 1.75 milligrams/kg per hour continuous IV infusion (FDA approved for use in cardiac catheterization laboratory only) | Optimal anticoagulation strategies are not completely defined. |
| Peripheral Artery Disease | |
| Aspirin 162–325 milligrams PO once a day | — |
| Cilostazol 100 milligrams PO twice a day | — |
| Acute Ischemic Stroke | |
| Alteplase 0.9 milligram/kg (maximum, 90 milligrams) with 10% of total dose given as an IV bolus over 1 min followed by the remainder as an IV infusion over 60 min | Use of fibrinolytics in acute ischemic stroke requires strict adherence to recommended guidelines and should be done with informed consent. Adjunctive use of anticoagulants should be avoided for 48 h. |
| Postischemic Stroke | |
| Aspirin 81 milligrams PO once a day | — |
| Clopidogrel 75 milligrams PO once a day | Use clopidogrel if aspirin allergic. |
| Dipyridamole 200 milligrams extended-release PO twice a day | Usually combined with aspirin 25–50 milligrams PO twice a day. |
| Transient Ischemic Attack | |
| Aspirin 81 milligrams PO once a day | Use clopidogrel if “aspirin failure” or aspirin allergic. |
| Clopidogrel 75 milligrams PO per day | — |
| Stroke Prevention in Atrial Fibrillation | |
| Warfarin dose monitored and adjusted by INR | Target INR is 2.0–3.0 in most patients. |
| Dabigatran 75–150 milligrams PO twice daily | Dosing guided by renal function; not routinely monitored. |
| Rivaroxaban 15–20 milligrams PO once a day | Dosing guided by renal function; not routinely monitored. |
| Apixaban 2.5–5.0 milligrams PO twice daily | Dose adjustment in patients with any two of the following characteristics: Age >80 years Weight <60 kg Serum creatinine >1.5 mg/dL |
Warfarin, a hydroxy coumarin compound, is the most widely used oral anticoagulant.1,2 Warfarin is readily absorbed after ingestion, reaching peak blood concentrations in 2 to 4 hours, and has a circulating half-life of 20 to 60 hours. Warfarin is bound to albumin, metabolized by the liver, and excreted in the urine. Warfarin blocks activation of vitamin K and thereby interferes with hepatic carboxylation of coagulation factors II, VII, IX, and X. The decrease in these vitamin K–dependent cofactors impairs the extrinsic and common coagulation pathway. Warfarin also blocks the synthesis of proteins C and S. Activated protein C (with protein S and phospholipid as cofactors) proteolyses factors Va and VIIIa, thereby inhibiting the coagulation cascade. Thus, warfarin has both an antithrombotic effect (by inhibiting the synthesis of factors II, VII, IX, and X) and a prothrombotic effect (through inhibition of proteins C and S production), but during maintenance therapy, the overwhelming effect is one of anticoagulation.
Warfarin dosing is guided by measurement of the International Normalized Ratio (INR), a standardized measurement of prothrombin time, with a desired therapeutic range of 2.0 to 3.0 in most cases.2 Drugs and food that interfere with warfarin absorption, bind to albumin, or alter hepatic metabolism can have a profound effect on warfarin activity (Table 239-2). Warfarin is generally contraindicated in pregnancy because it is teratogenic (especially during the 6th to 12th week of gestation) and can cause fetal hemorrhage.
| Consideration | Effect on PT or INR* |
|---|---|
| Major | |
| Vitamin K malabsorption or dietary deficiency | ↑ |
| Excess vitamin K | ↓ |
| Reduced gut bacteria (antibiotics)† | ↑ |
| Decreased warfarin absorption | ↓ |
| Altered warfarin metabolism (cytochrome P-450) | Variable |
| Drug effects† | Variable |
| Other | |
| Decreased clotting factor production (liver disease) | ↑ |
| Increased metabolism of clotting factors (fever) | ↓ |
| Confounding technical or laboratory factors (e.g., phlebotomy, handling in transport, thromboplastin reagents) | Variable |
Protein C has a short half-life (8 hours), and plasma levels quickly fall after starting warfarin. The vitamin K–dependent coagulation factors have half-lives that range from approximately 7 hours for FVII to approximately 60 hours for prothrombin (FII). The phase delay between the fall in levels of protein C (an antithrombotic protein) and the fall in levels of the four affected coagulation factors (prothrombotic proteins) results in a transient state of increased thrombogenesis at the start of warfarin therapy that persists for 24 to 36 hours. This hypercoagulable state is mitigated by providing sufficient overlap with a parenteral anticoagulant (e.g., heparin) during the first 3 to 5 days of warfarin treatment1,2 and during any interruption of warfarin therapy for surgery or an invasive procedure.3,4,5 Because factors X and II have relatively long half-lives, the parenteral anticoagulant should not be discontinued until the INR is in the desired therapeutic range for 2 consecutive days. Thus, a noncompliant patient with the risk for catastrophic complications from sudden intravascular thrombosis—such as a patient with a mechanical prosthetic heart valve who has stopped oral anticoagulants—should be treated with a parenteral anticoagulant in addition to restarting warfarin.
There is also a prothrombotic rebound during the first 4 days after cessation of warfarin therapy. However, there is no increased incidence of clinical episodes of thrombosis with termination of warfarin therapy, and thromboembolic events that occur in patients after warfarin discontinuation are most-likely related to the underlying condition.
The two major complications of warfarin therapy are bleeding and skin necrosis. The most important factor influencing the risk of bleeding is the intensity of anticoagulant therapy. The risk of clinically significant bleeding is increased when the INR is >4.5 to 5.0.1,2 Skin necrosis occurs primarily (but not exclusively) in patients with protein C deficiency. This complication usually develops 3 to 8 days after starting treatment and is caused by thrombosis of small cutaneous vessels. Treatment includes discontinuation of warfarin, administration of a parenteral anticoagulant to maintain desired anticoagulation, vitamin K1 administration, and screening for protein C and S deficiencies.
Patient-specific risk factors for increased risk of bleeding during warfarin treatment include hypertension, anemia, prior cerebrovascular disease, GI lesions, and renal disease. The relationship between advanced age and warfarin-associated bleeding is controversial. Elderly individuals who are otherwise appropriate candidates for anticoagulant therapy should not have warfarin withheld solely because of their age, although elderly patients require more frequent and careful monitoring. Medications that increase warfarin activity and antiplatelet medications increase bleeding risk during warfarin therapy (Table 239-2).
Drug interactions with warfarin are numerous and complex. Emergency physicians should carefully review medications prescribed on ED discharge, and it is recommended that drug–drug interaction references be utilized whenever adding a new medication to a patient on warfarin. Drugs frequently prescribed upon ED discharge should generally be avoided in patients on warfarin because of the increased risk of bleeding, including nonsteroidal anti-inflammatory drugs, sulfa-containing drugs (e.g., sulfamethoxazole), macrolides (with the exception of azithromycin), and fluoroquinolones. Drugs that induce hepatic cytochrome P-450 activity may increase the metabolism and reduce the effect of warfarin. Because the effect may take several days to manifest, the following agents should only be prescribed upon ED discharge after careful review and with close follow-up: barbiturates, anticonvulsants (e.g., phenytoin, carbamazepine, primidone), antibiotics (e.g., dicloxacillin, nafcillin, rifampin), and antipsychotics or sedatives (e.g., haloperidol, trazodone).
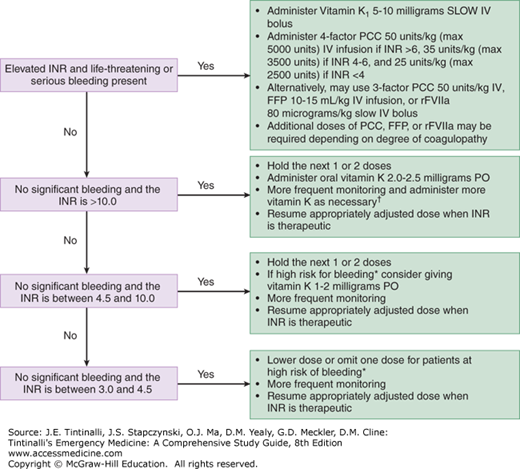
The two important principles when warfarin-treated patients bleed with a prolonged INR are (1) attempt to identify and attenuate the cause of bleeding, and (2) lower the intensity of the anticoagulant effect. In patients with a modestly elevated INR without clinically evident bleeding, cessation of warfarin, careful observation, and periodic monitoring comprise the safest course (Figure 239-1).2,6,7 Conversely, reversal is recommended when the INR is markedly elevated or there is clinically significant bleeding.2,7 The speed and extent of reversal should be balanced against the risk of recurrent thromboembolism in patients who require therapeutic anticoagulation. For example, an over-anticoagulated patient with a prosthetic mitral valve may develop fatal thrombosis if supratherapeutic anticoagulation is rapidly and fully reversed.
FIGURE 239-1.
Management of prolonged INR (warfarin-induced coagulopathy). *High risk of bleeding: age >75 years, concurrent antiplatelet drug use, polypharmacy, liver or renal disease, alcoholism, recent surgery, or trauma. †There are no validated tools to predict risk of short-term major bleeding in patients with severe over-anticoagulation. The decision to admit for observation relies on physician judgement. FFP = fresh frozen plasma; 4-factor PCC = Prothrombin Complex Concentrate containing coagulation factors 2, 7, 9, and 10; 3-factor PCC = Prothrombin Complex Concentrate containing coagulation factors 2, 9, and 10; rFVIIa = recombinant activated factor VII.
Three approaches used to reverse warfarin-induced coagulopathy are as follows: (1) stop warfarin therapy; (2) administer vitamin K1 (PO or IV); and (3) replace deficient coagulation factors using either fresh frozen plasma, 3- for 4-factor prothrombin complex concentrate, or recombinant activated factor VII (Figure 239-1).2,8,9,10,11 In asymptomatic patients with an elevated INR of 4.5 to 10 due to warfarin, oral vitamin K1 1.0 to 2.0 milligrams will produce a measurable reduction in INR by 16 hours, with a therapeutic level by the second day.6,7 In asymptomatic patients with an INR >10, oral vitamin K1 2 milligrams is also effective, although the reduction in INR takes longer. Although low-dose oral vitamin K1 carries a small risk for patients who require therapeutic anticoagulation, it is recommended that the emergency physician consult an appropriate specialist before using vitamin K1 to reverse anticoagulation in stable patients.
IV vitamin K1 carries a rare but serious, non–dose-dependent risk of anaphylaxis and should not be used for routine reversal of therapeutic over-anticoagulation. For patients who require continued anticoagulation, IV administration also carries the risk of overcorrection not associated with oral use. IV vitamin K1 should be restricted to those patients with life-threatening bleeding2 and to symptomatic patients poisoned by an excessive ingestion of warfarin (e.g., suicidal overdose). Generally, overdose patients do not require long-term therapeutic anticoagulation, and reversal does not carry the risk of recurrent thrombosis.
The fastest method of reversing therapeutic over-anticoagulation is with coagulation factor infusion, using either fresh frozen plasma, prothrombin complex concentrates, or recombinant activated factor VII.8,9,10,11 For patients with intense anticoagulation (INR >10) who require only partial reversal, fresh frozen plasma 10 to 15 mL/kg would be expected to restore coagulation factors to about 30% of normal, corresponding to an INR of 1.7 to 1.8. Disadvantages of fresh frozen plasma include potential fluid overload, which can be difficult to reverse with furosemide. Some institutions provide “universal donor” fresh frozen plasma, which is derived from AB+ donors and thus contains no AB antibodies. If available and indicated, it can be given without a type and cross-match of blood of the recipient and as soon as it is defrosted (20 to 30 minutes).
For patients with life-threatening hemorrhage who require rapid, complete reversal, such as warfarin-associated intracranial hemorrhage, prothrombin complex concentrates or recombinant activated factor VII are preferred.12,13,14 Four-factor prothrombin complex concentrate is preferred over the 3-factor product, and dosing is according to the patient’s INR level; expert consultation is advised prior to administration. Additional doses of these products may be required depending on the degree of coagulopathy.
Dabigatran etexilate, an oral direct thrombin inhibitor, is used to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation, and the risk of recurrent DVT or PE after treatment of the acute episode.1,15,16,17,18 After ingestion, dabigatran etexilate is converted to the active agent dabigatran by esterase, achieving peak serum concentrations in 2 hours with a terminal elimination half-life of 12 to 17 hours. Dabigatran is a reversible inhibitor of both circulating and clot-bound thrombin. Dabigatran has more predictable pharmacologic activity than warfarin, a broad therapeutic window, low interpatient variability, and no significant drug–drug (except for rifampin) or drug–diet interactions. Monitoring with standard coagulation tests during therapeutic use is not required. In routine use in patients with atrial fibrillation, dabigatran is generally safer than warfarin, with the notable exception of a higher risk of major GI bleeding. As with warfarin, the concomitant use of nonsteroidal anti-inflammatory drugs and other antiplatelet medications greatly increases the risk of bleeding for patients taking dabigatran.
Prothrombin time and the aPTT are insensitive to the activity of dabigatran, whereas the thrombin clotting time is typically overly sensitive.1,19 The ecarin clotting time has a linear dose response through the range of dabigatran concentrations seen during clinical use, but this test is not commonly available. For practical purposes, a normal thrombin clotting time excludes a significant coagulopathy due to dabigatran.1
Only about 15% to 20% of absorbed dabigatran is metabolized, and the remainder is excreted unchanged in the urine. It is therefore important to maintain urinary output in patients with active bleeding while taking dabigatran to enhance drug elimination. If emergency measures are required to reverse the anticoagulant effect of dabigatran, hemodialysis can be effective with removal of 60% or more of the drug within 2 hours.1,11,20
Idarucizumab, a monoclonal antibiotic fragment that binds dabigatran, is in development to reduce serious bleeding or reverse anticoagulation in patients who require an urgent procedure in patients taking dabigatran.20 Until idarucizumab is available, observational experience suggests that both recombinant activated FVII (rFVIIa) or activated prothrombin complex concentrate (aPCC) can reverse the anticoagulative effect of dabigatran,21,22 whereas fresh frozen plasma does not.1
Rivaroxaban and apixaban, oral direct FXa inhibitors, are used for the prevention of venous thromboembolism in adult patients undergoing elective hip or knee replacement surgery and for the reduction of the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation.1,23 Edoxaban, a third orally active direct FXa inhibitor is likely to become available soon for similar indications.24 These FXa inhibitors have predictable pharmacologic properties and do not require routine laboratory monitoring.
The effect of rivaroxaban or apixaban on blood clotting is not easy to reliably measure. Although FXa inhibitors demonstrate a dose-dependent increase in prothrombin time, the sensitivity of this assay varies according to the thromboplastin reagent used. The activated partial thromboplastin time is less sensitive than the prothrombin time for measuring effects of FXa inhibitors. The currently available anti–FXa activity assays must be adjusted for the specific FXa inhibitor used.
Rivaroxaban and apixaban are metabolized by the liver, with about two thirds of the drug and metabolites excreted by the kidneys with an elimination half-life of 5 to 12 hours in healthy individuals. Discontinuation of the drug for at least 24 hours could be sufficient when there is no imminent need for reversal, as in elective or nonurgent procedures. However, in patients with renal impairment and the elderly, additional time for clearance would be required before any surgical procedures are undertaken. There is an increased risk of stroke or other thrombotic event when rivaroxaban is suddenly discontinued in patients with nonvalvular atrial fibrillation.25 When possible, another anticoagulant should be substituted.
Andexanet alfa, a recombinant modified FXa, is in development for treatment of major bleeding due to FXa inhibitors, but is currently not available for clinical use. Due to high plasma protein binding, rivaroxaban or apixaban are not readily removed by dialysis. Current options for the emergency reversal include transfusion of blood products such as fresh frozen plasma, prothrombin concentrate complex, or recombinant activated factor VII.1,23,26
Administration of activated charcoal within 2 hours of taking an oral anticoagulant may adsorb the drug from the intestines before it reaches the plasma. It would be reasonable to administer activated charcoal to a patient who has deliberately or accidentally ingested an inappropriate amount of dabigatran, rivaroxaban, or apixaban, and likewise in a patient with significant bleeding who has recently ingested even a therapeutic dose.
HEPARINS AND POLYSACCHARIDES
Unfractionated heparin (UFH) is a heterogeneous mixture of polysaccharides ranging in molecular weight from 3 to 30 kD, with most commercial preparations possessing a mean molecular weight of about 15 kD, corresponding to about 45 saccharide units.27,28 The anticoagulant effect of UFH requires binding to antithrombin (previously named antithrombin III), and heparins are therefore “indirect” anticoagulants.27,28 Although the UFH–antithrombin complex interferes with several activated factors in both the extrinsic and common coagulation pathways (Xa, IXa, XIa, and XIIa, and thrombin), the primary anticoagulant effect of heparin is due to thrombin and FXa inhibition. The majority of heparin’s anticoagulant effect is dependent on a unique pentasaccharide sequence found in only about one third of heparin molecules. Variations in polysaccharide chain lengths found in UFH likely contribute to the unpredictable nature of heparin’s dose-response relationship.
UFH is given parenterally with a half-life (30 to 150 minutes) that depends on the dose and route. Weight-based IV UFH dosing protocols are the most reliable approach for achieving a therapeutic effect and preventing further thrombosis during acute thromboembolic events.28 Subcutaneous UFH heparin is not recommended for the treatment of acute thromboembolic disease because the bioavailability via this route of administration ranges from 10% to 90%, depending on the dose. However, subcutaneous UFH can be used to prevent thromboembolism (Table 239-1). Because UFH interferes with most laboratory investigations for hypercoagulable states, these tests should ideally be ordered before the patient is anticoagulated. Neither UFH nor low-molecular-weight heparin crosses the placenta; consequently, both are safe to use in pregnancy.
UFH has an unpredictable anticoagulation effect, requires frequent monitoring, binds nonproductively to vascular endothelium and ubiquitous plasma proteins, and actually activates platelets by interacting with platelet factor 4. The unpredictable inhibition of thrombin by heparin is attributable to a low bioavailability from extensive nonspecific binding to serum proteins, macrophages, and endothelial cells. The anticoagulant effect of UFH is generally monitored with the activated partial thromboplastin time (Table 239-1).28 For most purposes, a therapeutic range for UFH can be either an activated partial thromboplastin time of 1.5 to 2.5 times the “normal” value, a heparin level of 0.2 to 0.4 units/mL when assayed by protamine titration, or a level of 0.3 to 0.7 units/mL when measured for anti–FXa activity. UFH can increase the prothrombin time by a variable amount, typically 1 to 5 seconds depending on the heparin concentration and the thromboplastin reagent used in the assay.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree