Thoracentesis
Mark M. Wilson
Richard S. Irwin
Thoracentesis is an invasive procedure that involves the introduction of a needle, cannula, or trocar into the pleural space to remove accumulated fluid or air. Although a few prospective studies have critically evaluated the clinical value and complications associated with it [1, 2 and 3], most studies concerning thoracentesis have dealt with the interpretation of the pleural fluid analyses [4, 5].
Indications
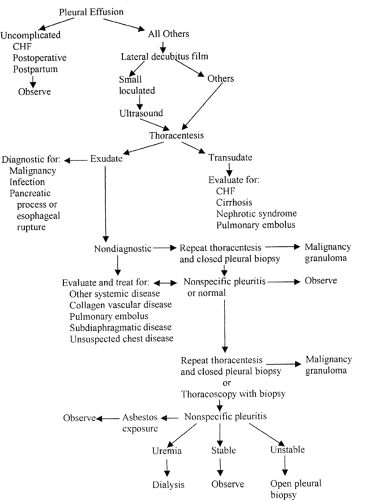
Although history (cough, dyspnea, or pleuritic chest pain) and physical findings (dullness to percussion, decreased breath sounds, and decreased vocal fremitus) suggest that an effusion is present, chest radiography or ultrasonic examination is essential to confirm the clinical suspicion. Thoracentesis can be performed for diagnostic or therapeutic reasons. When done for diagnostic reasons, the procedure should be performed whenever possible before any treatment has been given to avoid confusion in interpretation [6]. Analysis of pleural fluid has been shown to yield clinically useful information in more than 90% of cases [3]. The four most common diagnoses for symptomatic and asymptomatic pleural effusions are malignancy, congestive heart failure, parapneumonia, and postoperative sympathetic effusions [7]. A diagnostic algorithm for evaluation of a pleural effusion of unknown etiology is presented in Figure 10-1. In patients whose pleural effusion remains undiagnosed after thoracentesis and closed pleural biopsy, thoracoscopy should be considered for visualization of the pleura and directed biopsy. Thoracoscopy has provided a positive diagnosis in more than 80% of patients with recurrent pleural effusions that are not diagnosed by repeated thoracentesis, pleural biopsy, or bronchoscopy [8].
Therapeutic thoracentesis is indicated to remove fluid or air that is causing cardiopulmonary embarrassment or for relief of severe symptoms. Definitive drainage of the pleural space with a thoracostomy tube must be done for a tension pneumothorax and should be considered for pneumothorax that is slowly enlarging or the instillation of a sclerosing agent after drainage of a recurrent malignant pleural effusion [9].
Contraindications
Absolute contraindications to performing a thoracentesis are an uncooperative patient, the inability to identify the top of the rib clearly under the percutaneous puncture site, a lack of expertise in performing the procedure, and the presence of a coagulation abnormality that cannot be corrected. Relative contraindications to a thoracentesis include entry into an area where known bullous lung disease exists, a patient on positive end expiratory pressure, and a patient who has only one “functioning” lung (the other having been surgically removed or that has severe disease limiting its gas exchange function). In these settings, it may be safer to perform the thoracentesis under ultrasonic guidance.
Complications
A number of prospective studies have documented that complications associated with the procedure are not infrequent [1, 2, 10]. The overall complication rate has been reported to be as high as 50% to 78% and can be further categorized as major (15% to 19%) or minor (31% to 63%) [2, 3]. Complication rates appear to be indirectly related to experience level of the operator; the more experienced, the fewer the complications [11]. Although death due to the procedure is infrequently reported, complications may be life threatening [1].
Major complications include pneumothorax, hemopneumothorax, hemorrhage, hypotension, and reexpansion pulmonary edema. The reported incidence of pneumothorax varies between 3% and 30% [1,2 and 3, 11,12,13 and 14], with up to one third to one half of those with demonstrated pneumothoraces requiring subsequent intervention. Various investigators have reported associations between pneumothorax and underlying lung disease (chronic obstructive pulmonary disease, prior thoracic radiation, prior thoracic surgery, lung cancer) [10, 12, 13, 15], needle size and technique [3, 15], number of passes required to obtain a sample [12, 15], aspiration of air during the procedure [12], operator experience [1, 3, 10, 11], use of a vacuum bottle [13], size of the effusion [2, 15], and mechanical ventilation versus spontaneously breathing patients [16]. Some of the above-mentioned studies report directly contradictory findings compared to other similar studies. This is most apparent in the reported association between pneumothorax and therapeutic thoracentesis [3, 10, 15], which was not supported by subsequent large prospective trials [13, 14]. The most likely explanation for this discrepancy in the literature concerning the presumed increased risk for pneumothorax for therapeutic over diagnostic procedures is the generally lower level of operator experience in the first group. Small sample sizes also limit the generalization of reported findings to allow for the delineation of a clear risk profile for the development of a pneumothorax due to thoracentesis. The presence of baseline lung disease, low level of operator experience with the procedure, and the use of positive-pressure mechanical ventilation appear for now to be the best-established
risk factors in the literature. Further research involving more patients is needed.
risk factors in the literature. Further research involving more patients is needed.
Although pneumothorax is most commonly due to laceration of lung parenchyma, room air may enter the pleural space if the thoracentesis needle is open to room air when a spontaneously breathing patient takes a deep breath (intrapleural pressure is subatmospheric). The pneumothorax may be small and asymptomatic, resolving spontaneously, or large and associated with respiratory compromise, requiring chest tube drainage. Hemorrhage can occur from laceration of an intercostal artery or inadvertent puncture of the liver or spleen, even if coagulation studies are normal. The risk of intercostal artery laceration is greatest in the elderly because of increased tortuosity of their vessels [17]. This last complication is potentially lethal, and open thoracotomy may be required to control the bleeding.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree