Fig. 38.1
(a) Alexis Carrel, (b) Richard Lillehei (left) and William Kelly (right), (c) Thomas Starzl
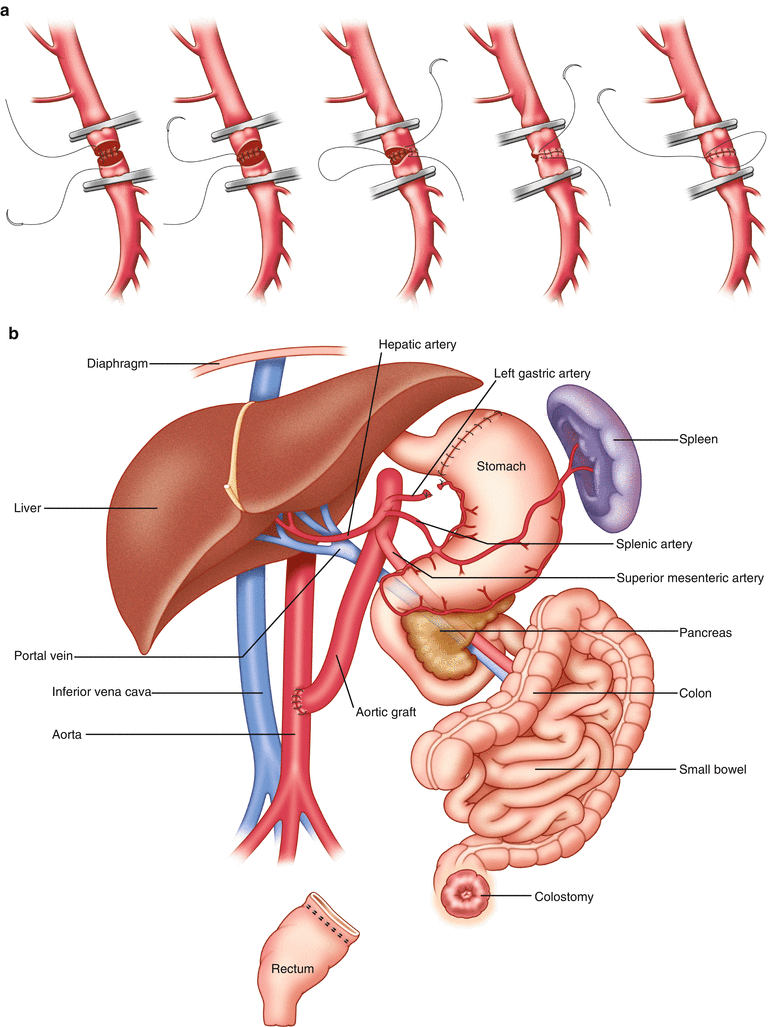
Fig. 38.2
(a) Technique for anastomosing the superior mesenteric vessels [5]. (b) Schematic view of the transplanted tissues and their anatomic relation to the host
The Carrel’s successful implantation of vascular grafts and performance of several autotransplantations were behind the landmark initial experiment of Lillehei and his colleagues at the University of Minnesota. The designed animal model assessed the physiological response of different degrees of small bowel ischemia. The technical feasibility of re-implantation as an auto or visceral allograft was also examined with special focus on patency of the venous and arterial vasculature [5].
The Starzl’s model of “mass homotransplantations of abdominal organs” was introduced to study the behavior of a large denervated homograft in which the lymphatic drainage was interrupted. The boldness of the concept was evident in the cataclysmic postoperative course with a longest survival of 9 days among 19 dogs. However, the experiment observed a great degree of functional preservation of the liver that suggests mitigation of the rejection process. The same observation has been recently documented in humans by the senior author [4, 7–9].
Visceral Transplantation in Humans
Isolated Intestine Transplantation
The successful development of clinical intestinal and multivisceral transplantation is one of the most important milestones in modern history of organ transplantation. Five years after the Lillehei experiment, Deterling at the Boston Floating Hospital [10] performed the first small bowel transplant in an infant by using a segment of the mother’s ileum. Another intestinal transplant in a child was also declared for the first time by Deterling during the discussion of Alican’s first clinical case at the eleventh annual meeting of the society for surgery of the alimentary tract in 1970 [10]. With azathioprine (Imuran) being the primary immunosuppressive agent, the attempts of these innovative surgeons and others across the globe (Fig. 38.3) were short lived with a patient survival ranging from 12 h to a few weeks (Table 38.1) [10–14].
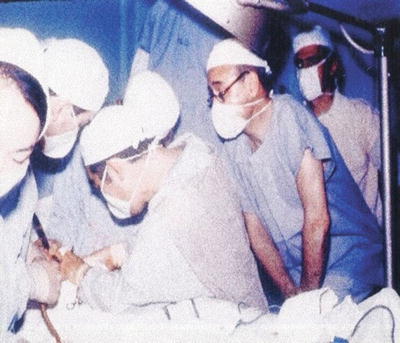

Fig. 38.3
Masayuki Okumura performing the first small bowel transplant in Latin America at the University Hospital of Sao Paulo Brazil in 1968
Table 38.1
Clinical intestinal transplantation in the azathioprine era
Year | Author | Institution | Etiology of intestinal failure | Graft survival |
|---|---|---|---|---|
1964 | Deterling [10] | Boston Floating Hospital | Mesenteric thrombosis | 12 h |
1964 | Deterling [10] | Boston Floating Hospital | Mesenteric thrombosis | 2 Days |
1967 | Lillehei [11] | University of Minnesota | Intestinal infarction | A few hours |
1968 | Okumura [12] | University Hospital-Sao Paulo Brazil | Mesenteric thrombosis | 10 Days |
1969 | Olivier [14] | Hôtel-Dieu de Paris | Gardner’s syndrome | 23 Days |
1969 | Alican [10] | University of Mississippi | Strangulation by a mesenteric band | 9 Days |
1969 | Okumura [12] | University Hospital-Sao Paulo Brazil | Volvulus | 5 Days |
1970 | Fortner [13] | Memorial Sloan Kettering | Gardner’s syndrome | 79 Days |
With the late 1970s arrival of cyclosporine, further worldwide attempts were made in humans after good results in rodent animal models. With 13 publications in the English literature (Table 38.2) [13, 15–21], better survival was observed compared to the azathioprine era. Of these recipients, only one patient is currently alive with fully functioning graft for nearly 25 years.
Table 38.2
Clinical intestinal transplantation in the cyclosporine era
Year | Author | Institution | Etiology of intestinal failure | Graft survival |
|---|---|---|---|---|
1985 | Cohen [13] | Toronto General Hospital | Gardner syndrome | 10 Days |
1987 | Tattersall [15] | Rush University, Chicago, USA | Short bowel syndrome | 13 Days |
1987 | Goulet [16] | Hôpital Necker-Enfants Malades, Paris, France | Neonatal volvulous | 8 h |
1987 | Goulet [16] | Hôpital Necker-Enfants Malades, Paris, France | Volvulous | 6 Month |
1987 | Deltz [17] | University of Kiel, Federal Republic of Germany | Volvulous | 12 Days |
1988 | Goulet [16] | Hôpital Necker-Enfants Malades, Paris, France | Volvulous | 17 Months |
1988 | Grant [18] | University of Western Ontario, London, Canada | Intestinal pseudo-obstruction | 14 Days |
1988 | Deltz [19] | University of Kiel, Federal Republic of Germany | SMV thrombosis | 49 Month |
1989 | Goulet [20] | Hôpital Necker-Enfants Malades, Paris, France | Neonatal volvulous | |
1989 | Goulet [20] | Hôpital Necker-Enfants Malades, Paris, France | Neonatal volvulous | 2 Months |
1989 | Goulet [20] | Hôpital Necker-Enfants Malades, Paris, France | Neonatal volvulous | 24 Days |
1989 | Wallander [21] | University Hospital, Uppsala, Sweden | Aganglionosis | 8 Weeks |
1990 | Goulet [20] | Hôpital Necker-Enfants Malades, Paris, France | Intestinal atresia | 7 Months |
The clinical introduction of FK-506 (currently known as tacrolimus) in 1989 refueled the interest of the transplant community in the field of intestinal transplantation. The early successful outcome with the first isolated intestinal transplantation and subsequent cases under tacrolimus-steroid-based immunosuppression proved the technical feasibility and practicality of intestinal transplantation under tacrolimus as a powerful immunosuppressive agent [22]. The initial encouraging results and continual improvement in outcome will be further discussed under current status of the procedure.
Composite Visceral Transplant
Twenty years after his first successful canine multivisceral transplant experiment, Starzl performed the first multivisceral transplant in humans in 1983 with en bloc inclusion of the stomach, duodenum, pancreas, intestine, and liver [23]. His enthusiasm was stimulated by the clinical availability of cyclosporine as a better immunosuppressive drug. Despite the painful operative experience with the first case, the recipient of the second transplant survived more than 6 months with fully functioning graft to die from progressive post-transplant lymphoproliferative disease (PTLD) . Similar attempts were made worldwide under cyclosporine with a patient survival ranging from 7.5 to 66 months (Table 38.3) [23–26]. The procedure has been increasingly utilized in the tacrolimus era [4, 27].
Table 38.3
Clinical transplantation of composite visceral grafts
Year | Author | Institution | Etiology of intestinal failure | Graft survival |
|---|---|---|---|---|
1983 | Starzl [23] | University of Pittsburgh Medical Center | Short bowel syndrome + liver failure | A few hours |
1986 | Williams [25] | Rush-Presbyterian-St Luke’s Medical Center | Gastroschisis + liver failure | 4 Days |
1987 | Starzl [23] | University of Pittsburgh Medical Center | Neonatal volvulus + liver failure | 192 Days |
1988 | Williams [25] | Rush-Presbyterian-St Luke’s Medical Center | Volvulus + liver failure | 109 Days |
1988 | Grant [24] | University of Western Ontario | Short bowel syndrome | |
1989 | Margreiter [26] | Innsbruck Medical University | Cancer (head of the pancreas) | 8 Months |
Shortly before the clinical introduction of tacrolimus, Grant et al. published the first case of successful combined liver and intestinal transplantation under cyclosporine in humans [24]. To overcome the observed prohibitive risk of intestinal allograft rejection under cyclosporine, the Ontario group transplanted both the liver and intestine from the same donor to a recipient with normal native liver. Such a successful outcome combined with the clinical introduction of tacrolimus stimulated a wave of enthusiasm that increased the utilization of the different types of intestinal transplantation for patients with irreversible intestinal failure and complex abdominal pathology.
Evolution of Immunosuppression
The clinical introduction of tacrolimus ushered in a new era in the field of intestinal and multivisceral transplantation. Soon after the initiation of the clinical trial with tacrolimus and steroid-based immunosuppression (type I), most centers experienced prohibitive risk of allograft rejections. During such an exciting era, different novel approaches were also introduced due to the introduction of new immunosuppressive agents with new insights into the mechanism of allograft acceptance and transplant tolerance.
With more emphasis on the difficulty of clinical care rather than survival, induction therapy with cyclophosphamide and daclizumab was introduced as part of multiple-drug immunosuppression including different cellular and molecular targets (type II) (Fig. 38.3). With better control of rejection, the overall survival has improved at major centers and according to the Intestinal Transplant Registry (ITR) [4, 28–30]. Unfortunately, updated results confirmed the long-term detrimental effect of chronic multiple-drug maintenance immunosuppression with erosion of the observed early survival benefits beyond the 10-year post-transplant landmark [4] (Fig. 38.4a).
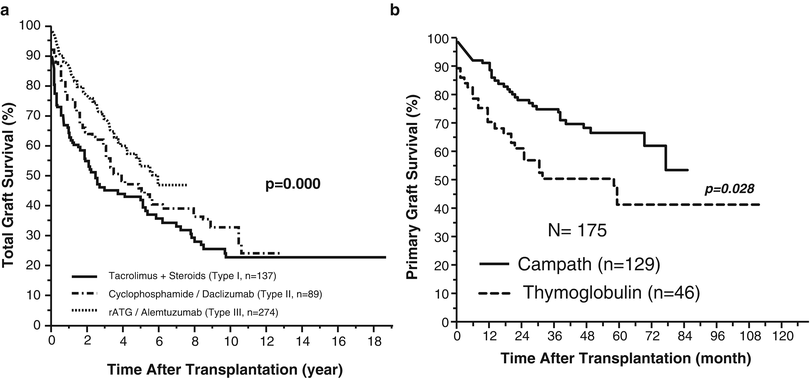

Fig. 38.4
(a) Improvement of visceral allograft survival according to the type of immunosuppression. (b) Better graft survival in patients pretreated with alemtuzumab (Campath-1H) compared to those pretreated with antithymocyte globulin (thymoglobulin) (data from Abu-Elmagd KM, Costa G, Bond GJ, et al. Five hundred intestinal and multivisceral transplantations at a single center: major advances with new challenges. Ann Surg 2009;250(4):567–81; and Abu-Elmagd KM, Costa G, Bond GJ, et al. A decade of experience with a single dose of rabbit antithymocyte globulin or alemtuzumab pretreatment for intestinal and multivisceral transplantation. Clin Transpl 2012:155–66)
With new insights into the mechanism of allograft acceptance and transplant tolerance, recipient preconditioning using thymoglobulin or alemtuzumab (Campath-1H) (Fig. 38.4b) with post-transplant minimal immunosuppression was introduced (type III) with the aim to improve allograft stability and reduce the need for long-term post-transplant immunosuppression at the University of Pittsburgh [31–33]. With perioperative partial depletion of the recipient lymphoid cells, amelioration of the initial donor-specific immune response is expected. Jointly application of minimal post-transplant immunosuppression has the potential to avoid the possible erosion of the alloengraftment mechanism of clonal exhaustion-deletion without high penalty of destructive immune response [32, 33]. The Pittsburgh intestinal and multivisceral recipients were the first to receive such a novel protocol with further improvement in overall outcome [4]. Reduction in the total incidence of intractable rejection and fatal infections partially contributed to better overall survival. Equally encouraging is the concomitant reduction in risk and fatality of PTLD despite the depletion of recipient lymphoid cells. With such a novel protocol, further improvement in outcome was achieved with more survival advantage utilizing alemtuzumab compared to rabbit antithymocyte globulin (thymoglobulin) (Fig. 38.5) [9]. A similar protocol has been reported by the Miami group utilizing alemtuzumab as an induction and not a pretreatment agent with multiple perioperative doses with no attempts to space out the tacrolimus maintenance dosage [34, 35].
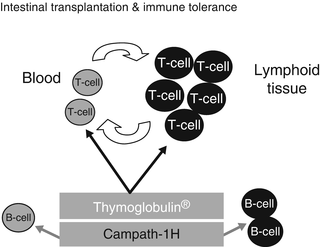

Fig. 38.5




This illustration depicts the dynamics of the lymphocyte depletion by both thymoglobulin (rATG) and Campath-1H (alemtuzumab). Note that both agents are effective in depleting both the intravascular and tissue T-lymphocytes. However, only Campath-1H is effective against the B-lymphocytes
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





