KEY POINTS
1. Inability to climb two flights of stairs showed a positive predictive value of 82% for postoperative pulmonary or cardiac complications.
2. Silent ischemia is more common in the elderly and diabetic patients, with 15% to 35% of all myocardial infarctions (MIs) occurring as silent events.
3. Isolated asymptomatic ventricular arrhythmias, even nonsustained ventricular tachycardia (VT), have not been associated with complications following noncardiac surgery.
4. Patients with left bundle branch block and especially with right coronary artery disease in whom a Swan-Ganz catheter is being placed may need availability of a transcutaneous pacemaker because of the risk of inducing right bundle branch block, and thus complete heart block, during passage of the pulmonary artery catheter.
5. Hypertension with blood pressure lower than 180/110 has not been found to be an important predictor of increased perioperative cardiac risk, but it may be a marker for chronic cardiovascular disease.
6. The presence of carotid stenosis increases the risk of postoperative stroke from approximately 2% in patients without carotid stenosis to 10% with stenosis of greater than 50%, and to 11% to 19% with stenosis of >80%.
7. Selective use of pharmacologic perfusion imaging in only patients who have at least one of two clinical risk factors for ischemic disease (age >70 yrs and congestive heart failure) can maximize the usefulness of this procedure in predicting cardiac outcome.
8. PCI is not beneficial when used solely as a means to prepare a patient with coronary artery disease for surgery.
9. Elective surgery requiring interruption of anti-platelet therapy should not be scheduled within 1 month of bare metal stent (BMS) placement or within 12 months of drug eluting stent (DES) placement.
10. Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers appear to cause perioperative hypotension, so it is prudent to hold these agents the morning of surgery but re-start them as soon as the patient is euvolemic postoperatively.

CARDIOVASCULAR DISEASE IS OUR SOCIETY’S number 1 health problem. According to the most recent CDC data, heart disease alone affects 26.8 million Americans, or about 12% of our population [1]. Of the 34 million Americans hospitalized in 2007, 3.9 million (11.5%) had heart disease. Heart disease remains the leading cause of death in patients greater than age 65, with an age adjusted death rate of 100 per 10,000, or 1%, per year. Furthermore, of the 45 million surgical procedures performed in the United States in 2007, approximately 6.9 million procedures involved the cardiovascular system. 405,000 were coronary artery bypass procedures, which represents a 13% decrease since 2003 and is associated with an increase in the number of percutaneous angioplasties and stents [2]. In contrast, the number of valve procedures has increased, with the number of valve repair procedures growing faster than the number of valve replacements [3]. The prime goals of preoperative evaluation and therapy for cardiac surgical procedures, therefore, are to quantify and reduce the patient’s risk during surgery and the postoperative period.
The factors that are important in determining perioperative morbidity and anesthetic management must be assessed carefully for each patient.
I. Patient presentation
A. Clinical perioperative risk assessment—multifactorial risk indices
Multifactorial risk indices, which identify and assign relative importance to many potential risk factors, have become increasingly sophisticated over the last three decades and are used with increasing frequency to combine multiple risk factors into a single risk estimate, to determine an individual patient’s risk of morbidity and mortality following heart surgery, to guide therapy, and to “risk adjust” the surgical outcomes of populations. One of the first multifactorial risk scores was developed by Paiement, in 1983 [4], and identified eight simple clinical risk factors:
1. poor left ventricular function
2. congestive heart failure
3. unstable angina or MI within 6 mos
4. age greater than 65 yrs
5. severe obesity
6. reoperation
7. emergency surgery
8. severe or uncontrolled systemic illness.
Recent models still incorporate many of these eight factors.
The preoperative clinical factors that affect hospital survival following heart surgery have been studied by multiple authors from the 1990s until the present time [3,5–9]. The initial studies focused on coronary artery bypass grafting (CABG) surgery, but more recent indices have been validated for valvular surgery and combined valve and CABG surgery as well.
The most important risk factors in these studies are compared in Table 3.1. The earlier indices assigned “point values” to indicate the relative risk of postoperative mortality associated with each preoperative risk factor, usually based on multivariate analysis. More recent studies provide more specific odds ratios of mortality associated with each of a larger number of predictors.
In 2001, Dupuis and colleagues developed and validated the cardiac risk evaluation (CARE) score, which incorporated similar factors but viewed them more intuitively in a manner similar to American Society of Anesthesiologists (ASA) physical status (Table 3.2) [9]. In 2004, Ouattara and colleagues [11] compared the CARE score to two other multifactorial indices, the Tu score [12] and Euroscore [5]. Their analysis found no difference among these scores in predicting mortality and morbidity following cardiac surgery at that time. However, in recent years the Society of Thoracic Surgeons continues to report more specific predictors and is updated annually to provide valuable risk data on CABG, valve, and combined procedures based on increasing volumes of cumulative data, making this perhaps the most robust of the risk indices.
1
B. Functional status. For patients undergoing most general and cardiac surgical procedures, perhaps the simplest and single most useful risk index is the patient’s functional status, or exercise tolerance. In major noncardiac surgery, Girish and colleagues found the inability to climb two flights of stairs showed a positive predictive value of 82% for postoperative pulmonary or cardiac complications [13]. This is an easily measured and sensitive index of cardiovascular risk, which takes into account a wide range of specific cardiac and noncardiac factors.
The level of exercise producing symptoms, as described classically by the New York Heart Association and Canadian Cardiovascular Society classifications, predicts the risk of both an ischemic event and operative mortality. During coronary revascularization procedures, operative mortality for patients with class IV symptoms is 1.4 times that of patients without preoperative congestive heart failure [14].
C. Genomic contributions to cardiac risk assessment. Genetic variations are the known basis for more than 40 cardiovascular disorders. Some of these, including familial hypercholesteremia, hypertrophic cardiomyopathy, dilated cardiomyopathy, and “channelopathies” such as the long QT syndrome, are known as monogenetic disorders, caused by alterations in one gene. These usually follow traditional Mendelian inheritance patterns, and their genetic basis is relatively easy to identify. Genetic testing is able to identify these diseases in up to 90% of patients before they become symptomatic, allowing prophylactic treatment and early therapy. For example, genetic identification of the sub-type of long QT syndrome can determine an affected patient’s risk of dysrhythmias associated with exercise, benefit from β–blockers, or need for Implantable Cardioverter Defibrillator (ICD) implantation [15].
Some of the areas of the greatest expansion of genomic medicine, however, are in its application to chronic diseases such as coronary artery and vascular disease. However, the causes of these disorders are multi-factorial, including environmental as well as genetic factors, and are at best due to complex interactions of many genes. Nevertheless, genetic information can be used to determine a patient’s susceptibility to disease, and this information can guide prophylactic therapy. Commercial tests are currently available for susceptibility to atrial fibrillation (AF) and MI. Genetic variants have been found which help to determine susceptibility to preoperative complications, including 4 which help to determine susceptibility to postoperative MI and ischemia. Similarly, genetic information may be used to determine variations in patient susceptibility to drugs, as for example a single allele variation can render patients much more susceptible to warfarin. Gene therapy may also target drug delivery to specific tissues [15].
Table 3.1 Multifactorial indices of cardiovascular risk for cardiac surgical procedures: summary of risk factors in recent multifactorial indices
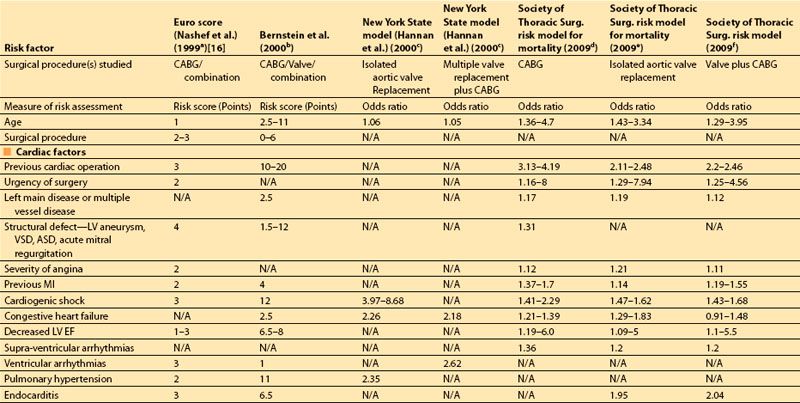
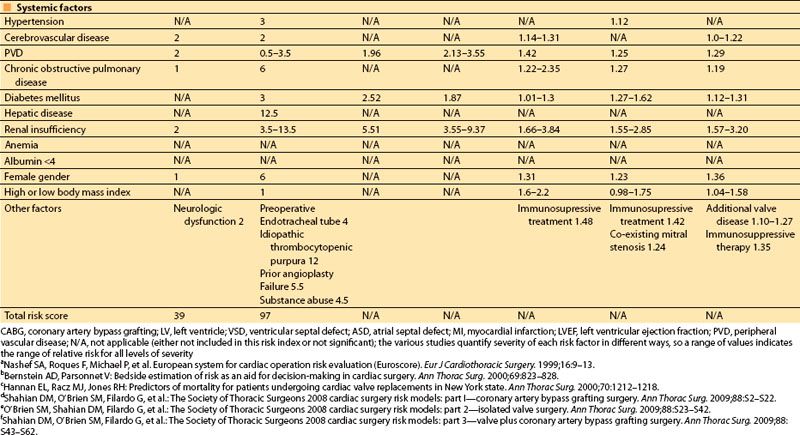
Table 3.2 Cardiac anesthesia risk evaluation (CARE)
D. Risk associated with surgical problems and procedures. The complexity of the surgical procedure itself may be the most important predictor of perioperative morbidity for many patients. Most, but not all, cardiac surgical procedures include the risks associated with cardiopulmonary bypass. Any procedure requiring cardiopulmonary bypass is associated with greater morbidity, caused by a systemic inflammatory response along with the risk of microemboli and hypoperfusion and most often involving the central nervous system, kidneys, lungs, and gastrointestinal tract. The extent of morbidity increases with the increased bypass duration.
Procedures on multiple heart valves, or on both the aortic valve and coronary arteries, carry a statistical morbidity much higher than that for procedures involving only a single valve or CABG alone. The mortality rate over the last decade for each procedure, for patients in the Society of Thoracic Surgeons database, is approximately 2.3% for CABG, 3.4% for isolated valve procedures, and 6.8% for valve procedures along with CABG [3,14,16].
II. Preoperative medical management of cardiovascular disease
A. Myocardial ischemia. In patients with known CAD, the most important risk factors that need to be assessed preoperatively are: (i) the amount of myocardium at risk; (ii) the ischemic threshold, or the heart rate at which ischemia occurs; (iii) the patient’s ventricular function or ejection fraction (EF); (iv) the stability of symptoms, because recent acceleration of angina may reflect a ruptured coronary plaque; and (v) adequacy of current medical therapy.
1. Stable coronary syndrome (stable angina pectoris). Chronic stable angina most often results from obstruction to coronary artery blood flow by a fixed atherosclerotic coronary lesion in at least one of the large epicardial arteries. In the absence of such a lesion, however, the myocardium may be rendered ischemic by coronary artery spasm, vasculitis, trauma, or hypertrophy of the ventricular muscle, as occurs in aortic valve disease.
Neither the location, duration, or severity of angina, nor the presence of diabetes or peripheral vascular disease (PVD), indicate the extent of myocardium at risk, or the anatomic location of the coronary artery lesions. Therefore, the clinician must depend on diagnostic studies, such as myocardial perfusion imaging (MPI), stress echocardiography, and cardiac catheterization to assist in establishing risk. Though some centers use cardiac computed tomography (CT) scanning for this purpose, and though it does have a high sensitivity for detecting coronary calcification and coronary artery disease, it currently still has a low specificity, so it cannot yet be recommended as a definitive test. In patients with chronic stable angina, a reproducible amount of exercise, with its associated increases in heart rate and blood pressure, often precipitates angina. This angina threshold, which can be determined on preoperative exercise testing, is an important guide to perioperative hemodynamic management. Stable angina often responds to medical therapy as well as to PCI. Patients are referred for CABG surgery when refractory to medical therapy and not candidates for PCI.
a. Principles of the medical management of stable angina [17]
(1) aspirin at 75 to 162 mg daily
(2) β-blockade as initial therapy when not contraindicated
(3) calcium antagonists or long-acting nitrates as second-line therapy, or as first-line therapy when beta blockade is contraindicated
(4) use of ACE inhibitors indefinitely in patients with left ventricle (LV) EF <40%, diabetes, hypertension, or chronic renal failure
(5) annual influenza vaccine
(6) reducing risk by:
(a) Lipid management—reduce low density lipoproteins to <100 mg/dL using diet, exercise, and statin therapy.
(b) Blood pressure control—reduce to less than 140/90 or to less than 130/80 for patients with diabetes or kidney disease, for patients with coronary disease initially treating with β–blockers and ACE inhibitors.
(c) Smoking cessation
(d) Diabetes control
(e) Weight loss
(f) Diet and exercise
2. Acute coronary syndrome (unstable angina pectoris). Sometimes called crescendo angina, or unstable coronary syndrome, this symptom complex usually presents as:
a. Rest angina, within the first week of onset
b. New onset angina markedly limiting activity, within 2 wks of onset
c. Angina which is more frequent, of longer duration, or occurs with less exercise.
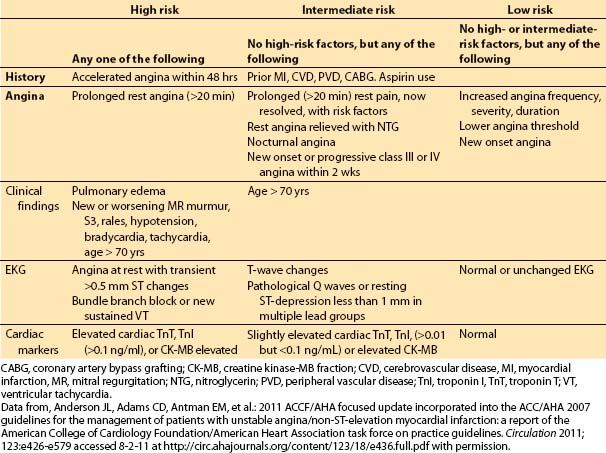
These symptoms often indicate rapid growth, rupture, or embolus of an existing plaque. Patients in this category have a higher incidence of MI and sudden death, and increased incidence of left main occlusion. The clinical factors important in determining the risk of MI or death in patients with unstable angina are shown in Table 3.3.
d. Medical management for acute coronary syndrome. Diagnostic and revascularization procedures are the central parts of the management for most patients with acute coronary syndrome. However, they are often accompanied or preceded by medical therapy. The medical management of unstable angina or of a non-ST segment elevation MI has two parts: (i) anti-ischemic therapy and (ii) antiplatelet and anticoagulant therapy. Medical anti-ischemic therapy depends largely on the presence or absence of ongoing ischemia and must be accompanied by an aggressive approach to secondary prevention or risk factor modification (Table 3.4 and Table 3.5).
3. Myocardial ischemia without angina may be manifested by fatigue, rapid onset of pulmonary edema, cardiac arrhythmias, syncope, or an “anginal equivalent,” most often characterized as indigestion or jaw pain. Silent ischemia is more common in the elderly and diabetic patients, with 15% to 35% of all MIs occurring as silent events, documented only on routine electrocardiogram (ECG). Whether related to coexisting disease or delayed therapy, silent ischemia has been associated with an unfavorable prognosis.
4. Prior MI interval between prior infarction and surgery.
In the non-cardiac surgical population, the occurrence of an MI within the 30 d before surgery is a significant preoperative risk factor [10]. Bernstein [6] assigns additional risk to an MI occurring 48 hrs before surgery and Eagle et al. [18] conclude that CABG has increased risk in patients with unstable angina, early postinfarction angina (within 2 days of a non-ST-Elevation Myocardial Infarction (non-STEMI) and during an acute MI), and that risk may be reduced by delaying CABG for 3 to 7 days after MI in stable patients. Coronary revascularization procedures, however, offer improved survival in patients with unstable angina and LV dysfunction.
Table 3.3 Risk factors for death or MI in patients with unstable angina
B. Congestive heart failure
1. Clinical assessment and medical management of heart failure. Ventricular dysfunction can occur almost immediately in association with an ischemic event. If no infarction occurs and the myocardium is reperfused, the ventricle recovers function quickly. Short episodes of ischemia followed by reperfusion may actually precondition the heart, so when it is exposed to more severe ischemia, the size and severity of MI is reduced. A MI may be associated with “stunned” myocardium, which recovers function within days to weeks, or “hibernating” myocardium, which may recover months after infarction and revascularization. Ventricular dysfunction and heart failure have been classified into four stages, A through D, based on cardiac structural changes and symptoms of heart failure. Management depends on the stage of the disease. ACE inhibitors and angiotensin II receptor blockers are usually used as first-line therapy, with the addition of β-blockers, aldosterone antagonists, diuretics, and implanted devices for more severely affected patients (Fig. 3.1).
2. Perioperative morbidity. Evidence of congestive heart failure or ventricular dysfunction preoperatively is associated with an increased operative mortality. Recent series summarized in Table 3.1 show a 1.5- to 2.5-fold greater risk of postoperative morbidity or mortality in patients with preoperative congestive heart failure, and a 1.4- to 12-fold greater risk in patients with preoperative cardiogenic shock.
In patients undergoing aortic valve replacement (AVR) for critical aortic stenosis and depressed EF, a cardiothoracic ratio of ≥0.6 is possibly the most important predictor of operative mortality, increasing the risk in some series more than 10-fold.
Table 3.4 Medical therapy for unstable angina: anti-ischemic therapy
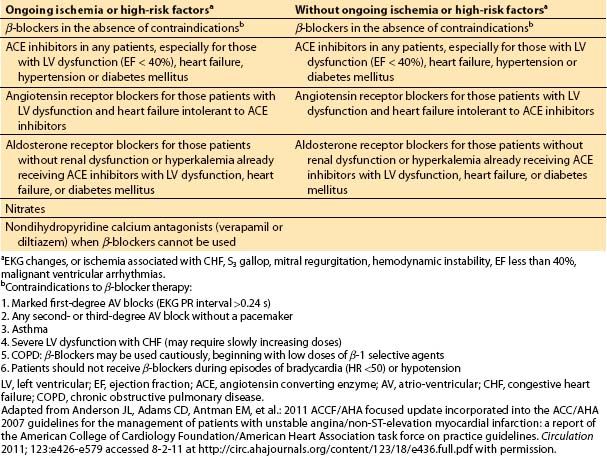
Table 3.5 Medical therapy for unstable angina and non-ST elevation MI: antiplatelet and anticoagulation therapy
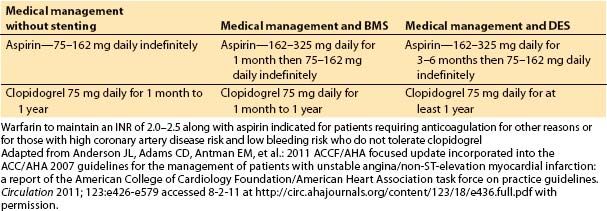
Figure 3.1 Functional classification and stages in the development of heart failure, and medical management of each stage. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; EF, ejection fraction; FHx CM, family history of cardiomyopathy; HF, heart failure; LV, left ventricular; LVH, left ventricular hypertrophy; and MI, myocardial infarction. [From Hunt SA, Abraham WT, Marshall HC, et al. 2009 Focused update incorporated into the ACC/AHA 2005 guideline update for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing committee to update the 2001 guidelines for the evaluation and management of heart failure). Circulation 2009;119:e391–e479]
C. Dysrhythmias
1. Incidence. Cardiac dysrhythmias are common in patients presenting for cardiac surgery, and become increasingly common with age after 50. In the perioperative period, abnormal rhythms occur in more than 75% of patients. However, those dysrhythmias are life-threatening in less than 1%.
2. Supraventricular tachycardia (SVT). SVTs appear most often in the preoperative history as palpitations or near-syncope. AF and flutter, the most common SVTs, increase in frequency with age and in association with organic heart disease. Preoperative patients with SVT who are hemodynamically stable are usually managed acutely with vagal maneuvers, adenosine, verapamil, or diltiazem to reduce heart rate and potentially convert the SVT back to sinus rhythm. Those with AF are in addition managed with anticoagulation to reduce stroke risk. However, in the last two decades, either surgical or catheter-based ablation have become more common, especially in patients unresponsive to medical therapy or in whom there may be structural abnormalities.
3. Ablation therapy
Catheter ablation using radiofrequency (RF) energy was first used in 1982. Medication can often control atrioventricular (AV) nodal reentrant tachycardia, However, when medications are ineffective, RF ablation can be performed. This procedure has a 97% success rate, 5% recurrence rate during the patient’s lifetime, and causes heart block requiring pacer therapy in 0.5% to 1% of patients in which it is used [19].
AF is the most frequent supraventricular arrhythmia. It can be caused by multiple reentrant circuits and also by a single focus in the SVC or pulmonary veins. Antiarrhythmic drugs can maintain sinus rhythm in one-half to two-third of patients with AF but may reduce the quality of life, decrease left ventricular function, and increase the risk of embolic complications.
The Maze procedure is a surgical procedure which disrupts the re-entrant pathways or ablates arrhythmogenic foci in the atria, often by isolating the ostia of the pulmonary veins. Thereby it converts the fibrillation to sinus rhythm, reducing the need for anticoagulation. However, this procedure is not always successful, and therefore a second type of procedure ablates the AV node, isolating the fibrillating atria from the ventricles, without attempting to prevent the AF itself. This procedure requires a permanent pacemaker to drive the ventricles, as well as ongoing anticoagulation to prevent thrombosis in the fibrillating atria.
The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) investigated the relative benefits of simple AF rate control versus conversion to sinus rhythm in patients older than age 65 [20]. Though the two treatments led to no difference in major bleeding, death, stroke, or quality of life, antiarrhythmic therapy and catheter ablation were recommended in symptomatic individuals in whom the arrhythmia interferes with their regular activities. A worldwide AF ablation survey reported 4550 of 8745 patients (52%) in sinus rhythm after ablation alone. With drug therapy, they reported a 75.9% success rate [21].
4. Ventricular arrhythmias and VT. Ventricular dysrhythmias have been classified according to clinical presentation (stable or unstable), type of rhythm (sustained or nonsustained VT, bundle-branch re-entrant or bidirectional VT, Torsades de pointes, ventricular flutter or fibrillation), or associated disease entity. Ventricular arrhythmias may lead directly to ventricular fibrillation and sudden cardiac death, especially if they occur in the setting of acute or recent infarction. However, isolated asymptomatic ventricular arrhythmias, even nonsustained VT, have not been associated with complications following noncardiac surgery. Patients with preoperative ventricular arrhythmias associated with left ventricular dysfunction and an EF < 30% to 35% are often managed with the prophylactic implantation of an ICD. Those not controlled with or not candidates for ICD therapy are managed medically with β-blockers as the first-line therapy. Amiodarone is the second-line drug used to prevent sudden cardiac death, with studies showing some survival benefit, and sotalol can also be effective though with greater proarrhythmic effects [22].
3
5. Bradycardia. Anesthetics frequently affect sinus node automaticity but rarely cause complete heart block. Asymptomatic patients with ECG-documented AV conduction disease (PR prolongation or single or bifascicular bundle branch block) rarely require temporary pacing perioperatively. However, symptomatic patients, or patients with Mobitz II or complete heart block, require preoperative evaluation for permanent pacing. Patients with a recent MI or with both first-degree AV block and bundle branch block may need temporary transvenous or transcutaneous pacing perioperatively (See Chapter 17—“Rhythm Management Devices”).
Patients with left bundle branch block in whom a Swan-Ganz catheter is being placed may need availability of a transcutaneous pacemaker because of the risk of inducing right bundle branch block, and thus complete heart block, during passage of the pulmonary artery catheter. Patients with a left bundle branch block and right CAD may be at particular risk during the passage of a Swan-Ganz catheter.
4
Patients with an indwelling cardiac pacemaker or ICD need to have their device identified and evaluated preoperatively. Special precautions need to be considered, as outlined in Chapter 17, to prevent untoward effects of electromagnetic interference in the operating room.
D. Hypertension
Systemic hypertension is one of the most common diseases of adulthood and is perhaps the most treatable cause of cardiovascular morbidity, including MI, stroke, PVD, renal failure, and heart failure. The contribution of hypertension to perioperative morbidity and the implications for anesthetic management depend on (i) blood pressure level, both with stress and at rest; (ii) the etiology of hypertension; (iii) pre-existing complications of hypertension; and (iv) physiologic changes due to drug therapy.
1. Blood pressure level. Data summarized in the JNC VII report in 2003 indicate that cardiovascular risk begins to increase at blood pressures above 115/75 and doubles with each increment of 20/10. Patients with blood pressures of 120–139/80–89 are considered prehypertensive and require drug therapy if they have associated diabetes or renal disease. Patients with blood pressures of 140–159/90–99 are considered hypertensive and all require chronic drug therapy. Those with blood pressures greater than 160/100, classified as stage 2 hypertensive, usually require combination drug therapy. Further, for patients older than 50 yrs, systolic blood pressure >140 is a much more important cardiovascular risk factor than diastolic blood pressure [23].
In contrast to the usual emphasis on the resting, unstimulated blood pressure in determining chronic medical management, preoperatively the patient’s blood pressure under stress, as in the preoperative clinic or holding area, may be a better predictor of their perioperative morbidity. Intraoperative cardiac morbidity in the form of dysrhythmias and ischemic ECG changes may be more frequent in Stage 3 hypertensive patients with awake systolic blood pressures greater than 180 mm Hg and diastolic blood pressures of greater than 110 mm Hg, and this morbidity may be reduced by preoperative treatment. In these patients the benefits of improving hypertensive control preoperatively should be weighed against the risks of delaying surgery. Blood pressure lower than 180/110 has not been found to be an important predictor of increased perioperative cardiac risk, but it may be a marker for chronic cardiovascular disease [10].
5
2. Etiology. The most common “primary” or “essential” hypertension is likely caused by a combination of multiple genetic and environmental factors, with the genetic contribution, at least, being irreversible. However, it is important preoperatively to exclude the 5% to 15% of patients with treatable causes of secondary hypertension, especially patients shown in Table 3.6. Common causes of secondary hypertension are usually renal, endocrine, or drug related, which account for an additional 5% to 10% of hypertensive patients. Other rare disorders are found in less than 1% of patients (Table 3.7). A laboratory investigation of secondary hypertension, when indicated, should include urinalysis, creatinine, glucose, electrolytes, calcium, EKG, and chest films. More extensive testing is usually not indicated unless blood pressure cannot be controlled or a high clinical suspicion exists [23]. Pheochromocytoma, although very rare, is particularly important because of its potential morbidity in association with anesthesia. Therefore, it should be ruled out preoperatively in patients with headache, labile or paroxysmal hypertension, abnormal pallor, or perspiration, even if delay of surgery is required.
Table 3.6 Risk factors for secondary hypertension

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








