KEY POINTS
1. Off-pump coronary artery bypass grafting (OPCAB) challenges the anesthesiologist and surgeon to maintain hemodynamic stability while delicate coronary arterial anastomoses are performed on a beating heart.
2. OPCAB has not produced the expected reductions in neurologic and renal complications, although it has consistently reduced perioperative blood loss and transfusion.
3. Adept positioning of the heart during OPCAB minimizes hemodynamic disturbances from reduced venous return, especially while performing coronary anastomoses within the right coronary and left circumflex arterial distributions. Maintaining adequate intravascular volume is essential.
4. Adjuncts such as intracoronary shunts, ischemic and/or anesthetic preconditioning, and intra-aortic balloon pumps may help to minimize ischemia during OPCAB. Circulatory support with vasoconstrictors and/or inotropic drugs is often required.
5. Robotic-assisted minimally invasive techniques can be used for coronary artery bypass grafting (CABG) performed either on- or off-cardiopulmonary bypass (CPB).
6. Transesophageal echocardiography (TEE) monitoring during OPCAB can promptly identify acute ischemia, although transgastric views are often compromised by the cardiac positioning required for distal coronary anastomoses.
7. Anesthetic techniques compatible with fast-tracking are most often used for OPCAB, which typically involves a “balanced” technique utilizing a combination of inhalational anesthetic, modest amounts of opioid, and intermediate-duration muscle relaxation. Excessive use of benzodiazepines and long-acting medications is avoided.
8. Minimally invasive cardiac valve surgery most often requires CPB, but the incisions are smaller and sometimes off the midline, and cannulation for CPB often utilizes port-access technology. Robotic-assisted techniques can be used for minimally invasive mitral valve replacement or repair.
9. TEE is critical during minimally invasive valve surgery (MIVS) for CPB cannulation and assessment of valve structure and function.
10. Percutaneous approaches to mitral regurgitation (Mitraclip), mitral stenosis (balloon mitral valvuloplasty), and aortic stenosis (transcatheter aortic valve implantation [TAVI]) are rapidly growing in popularity. Each approach presents unique challenges to the anesthesiologist; these procedures can be performed either with sedation or general anesthesia, each with its own benefits and risks.
11. Percutaneous valve procedures often involve transient profound hypotension from obstruction (e.g., balloon mitral valvuloplasty and predilation for TAVI) and either incidental or intentional dysrhythmias (e.g., rapid ventricular pacing for TAVI).
12. Hybrid operating rooms allow for interventional cardiology or radiology procedures to be performed in conjunction with open cardiac surgery including the use of CPB.
13. Transmyocardial laser revascularization (TMLR) may provide relief from ischemia through neovascularization and sympathetic denervation, although the exact mechanism remains unclear.
14. Patients undergoing TMLR typically have severe coronary artery disease, poor left ventricular (LV) function, and multiple coexisting diseases, making intraoperative anesthetic management challenging.
15. Intraoperative TMLR complications include gas embolization, major hemorrhage, acute decrease in ventricular function, injury to the mitral valve apparatus or conduction system, and atrial and ventricular arrhythmias.

THE PAST TWO DECADES HAVE witnessed a major evolution in cardiac surgery in parallel with “minimally invasive” and laparoscopic developments in other surgical fields [1]. Two major objectives have been a reduction in the use of CPB for revascularization and a reduction in the invasiveness of the surgical approach. The overall goals are to preserve and enhance the quality of the procedure(s) while providing faster recovery, reduced procedural costs, and reduced morbidity and mortality. The contribution of the anesthesia care team is to facilitate cost-effective early recovery while providing safe, excellent operating conditions both for the patient and the surgeon. Anesthetic techniques and monitoring modalities have needed to evolve with changes in surgical practice. Anesthesiologists have learned more about how to support the circulation during cardiac manipulation and periods of coronary occlusion. We have been charged with monitoring and support while the surgeon operates with minimal exposure while at the same time facilitating early recovery and discharge. The surgical techniques and their anesthetic considerations discussed in this chapter include the following: CABG without the use of CPB (off-pump CABG [OPCAB] and minimally invasive direct coronary artery bypass [MIDCAB]); MIVS, including TAVI; computer-enhanced, endoscopic robotic-controlled CABG; and TMLR, as an alternative revascularization technique. Although not mentioned subsequently, we recommend the routine use of intra-arterial blood pressure monitoring for all of these procedures because of the rapidity and frequency of significant hemodynamic disturbances and the need for frequent assessment of labs (e.g., arterial blood gases, activated clotting times, coagulation studies, etc.).
I. Off-pump coronary artery bypass (OPCAB) and minimally invasive direct coronary artery bypass
A. Historical perspective
1. Early revascularization surgery
a. Early attempts at coronary artery surgery without the use of CPB included the Vineberg procedure in Canada (tunneling the internal mammary artery [IMA] into the ischemic myocardium) in the 1950s, and internal mammary to coronary anastomosis in the 1960s by Kolessov in Russia.
b. Sabiston from the United States and Favolaro from South America reported the use of the saphenous vein for aorta-to-coronary artery bypass grafts, performed without CPB, in the same period.
c. The introduction of CABG in the late 1960s expanded the indications for CPB, which had enabled congenital heart repairs and heart valve surgery since the 1950s. CPB with the use of cardioplegia became the standard of care in the 1970s, providing a motionless field and myocardial “protection” with asystole and hypothermia.
2. Reports in the early 1990s
a. South American surgeons with limited resources continued to develop techniques for surgery without CPB, publishing in North American journals in the 1980s and early 1990s. In 1991, Benetti et al. [2] reported on 700 CABG procedures without CPB performed over a 12-yr period with very low morbidity and mortality.
b. North American and European interest grew in the 1990s, fueled by a desire to make surgery more appealing (vs. angioplasty) as well as the need to reduce cost and length of stay. Alterative incisions were explored, and techniques and devices to facilitate surgery on the beating heart were developed. The terms “OPCAB” and “MIDCAB” were coined.
3. Port-access (or “Heartport”)
a. Simultaneous with attempts to perform CABG without CPB, a group from Stanford University introduced a technique permitting surgery to be done with endoscopic instrumentation through small (1 to 2 cm) ports and a small thoracotomy incision. This was termed port-access surgery or by the trade name of Heartport (Johnson and Johnson, Inc., New Brunswick, NJ, USA). A motionless surgical field was required, necessitating CPB. Extensive use of TEE is required to assist in the placement of and to monitor the position of the various cannulae and the endoaortic balloon (see below).
b. Port-access cardiac surgery contributed new knowledge in two major areas: Percutaneous, endovascular instrumentation for CPB and instrumentation for performing surgery through a small thoracotomy incision. The latter techniques continue to be developed and modified to permit “minimally invasive” valve surgery through partial sternotomy or thoracotomy incisions.
4. MIDCAB. A number of alternative incisions to midline sternotomy have facilitated access to specific coronary artery distributions to allow CABG without CPB. The most popular alternative approach is the left anterior thoracotomy, which allows IMA harvest and grafting to the left anterior descending (LAD) artery territory. This is the procedure usually referred to as MIDCAB.
5. North American/European experience after 1998
a. Initially viewed by most as experimental, off-pump techniques are now established as an acceptable alternative to CABG with CPB. The reported use of OPCAB has been reported to be as high as 33% [3], but the range in practice is wide. Some surgeons perform virtually all revascularizations as OPCAB, which typically refers to a multivessel CABG performed through a median sternotomy without CPB. Most large cardiac surgery practices have at least one surgeon who performs a significant number of OPCAB procedures. The physiology and anesthetic management for OPCAB has been recently reviewed by Chassot et al. [4].
b. MIDCAB procedures are more technically demanding than OPCAB because they require specialized instrumentation and operating through a small incision. These procedures are done in a smaller number of institutions than OPCAB. Some surgeons harvest the IMA endoscopically before making the small incision to do the coronary anastomosis.
B. Rationale for avoiding sternotomy and cardiopulmonary bypass for coronary artery surgery
1
1. Reduction in complications
a. Sewing coronary vessels on the beating heart is technically challenging and not necessarily appropriate for all surgeons [5]. Whether or not there is a benefit of performing on-pump versus OPCAB is a topic of heated debate. Several published randomized trials [6–10] confirm reductions in enzyme release, bleeding, time to extubation, and length of stay. While there are long-term follow-up studies suggesting similar rates of survival and graft patency between the two groups [11,12], other studies suggest that graft patency is lower in off-pump procedures [9,13], with one large randomized controlled trial finding a higher 1-yr mortality rate in the off-pump group [13]. Of note, the latter studies came from surgeons less experienced in the off-pump technique. Intraoperative conversion from off-pump to on-pump has been associated with an increase in mortality [14,15]. Although reduction in stroke has been one of the proposed benefits of the technique (due to avoidance of aortic cannulation and cross-clamping), studies do not demonstrate this benefit. Similarly, reduction in renal dysfunction has been proposed but not proved in these studies and in one additional recent publication [16]. In August 2004, an updated guideline for CABG surgery was published by the American College of Cardiology and American Heart Association; this guideline recognizes the potential benefits for avoiding CPB but the need for further data with the lack of proved benefit in randomized controlled trials [17].
2
b. Avoidance of aortic manipulation and cannulation might reduce embolic complications such as stroke, yet a partial or side-biting aortic clamp may be necessary to perform proximal venous anastomoses in multivessel OPCAB. This can be avoided by using the IMA as the only proximal vessel with its origin intact or with the use of devices designed to avoid the use of a cross-clamp (e.g., the “Heartstring”).
c. The whole body “inflammatory response” induced by extracorporeal circulation is avoided with MIDCAB and OPCAB. This approach should result in lower fluid requirements and less coagulopathy and is consistent with lesser volumes of blood loss and transfusion demonstrated in several comparisons of OPCAB to CABG with CPB.
2. Competition with angioplasty. Refinements in interventional cardiology and reductions in postprocedure restenosis have allowed an ever-increasing population of patients to have coronary lesions treated in the catheterization laboratory, although long-term outcomes in multivessel coronary disease are slightly better with CABG than with stents. However, patients will often choose the less invasive interventional cardiology approach over surgery if those results are nearly equivalent. Evolution of surgical techniques to provide excellent results with less physiologic trespass may be necessary for coronary artery surgery to survive.
3. Progress toward truly “minimally invasive” surgery
a. Avoidance of CPB is more physiologically important than avoidance of sternotomy, but postoperative recovery from sternotomy is foremost in patients’ minds. The smaller the surgical scar, the better. The MIDCAB addresses this issue, but this approach can only access the LAD and its diagonal branches.
b. Cardiac surgeons have been slow to embrace endoscopic approaches partly because, until recently, existing technology did not provide the range of motion and control required for coronary artery anastomoses.
c. The port-access approach introduced endoscopic techniques to cardiac surgery; surgeons are now working with computer-assisted instruments to perform surgery on the beating heart (see later). Techniques developed for off-pump surgery are likely to contribute to the ability to perform such procedures endoscopically.
C. Refinement of surgical approach
1. Development of modern epicardial stabilizers
a. In early reports, compressive devices (e.g., metal extensions rigidly attached to the sternal retractor) were used to reduce the motion of the coronary vessel during the cardiac and respiratory cycles. These devices often interfered with cardiac function and were impossible to use for left circumflex coronary artery lesions.
b. Modern devices typically apply gentle pressure or epicardial suction, reducing the effect on myocardial function while providing better fixation of the area immediately surrounding the coronary artery anastomotic site. These devices also allow greater access to arteries on the inferior and posterior surfaces of the heart (Fig. 13.1).
Figure 13.1 The Octopus 2 tissue stabilizer (Medtronic Inc., Minneapolis, MN, U.S.A.). Through gentle suction the device elevates and pulls the tissue taut, thereby immobilizing the target area. (Courtesy of Medtronic Inc.)

2. Techniques to position the heart (through midline sternotomy)
a. Surgery on the anterior wall of the heart (LAD and diagonal branches) usually requires only mild repositioning, such as a laparotomy pad under the cardiac apex. This is associated with minimal effects on cardiac function.
b. Surgery on the right coronary artery (RCA) or the circumflex artery and its marginal branches requires turning or twisting of the heart. To do this manually (i.e., by an assistant) is cumbersome and is associated with hemodynamic compromise.
c. Use of posterior pericardial traction stitches and a gentle retracting “sock” (web roll wrapped around the apex in a “sling” to pull the heart to either side) greatly improves the hemodynamic tolerance of these abnormal positions.
d. For circumflex vessel distribution surgery, dissection of the right pericardium to prevent the right ventricle (RV) from being compressed as it is being turned allows preservation of hemodynamic function.
3. Surgical adjuncts to reduce ischemia
a. Performing CABG surgery on the beating heart requires a mandatory period of coronary occlusion for each distal coronary artery anastomosis.
b. Intracoronary shunts can maintain coronary flow at the possible cost of trauma to the endothelium.
4
c. “Ischemic preconditioning” involves a brief (e.g., one to four 5-min periods) occlusion and then the same period of reperfusion before performing the anastomosis. In animal models of myocardial infarction, this technique reduces the area of necrosis. A nearly equivalent physiologic effect can be provided by 1 MAC end-tidal isoflurane [18] or other inhaled agents, which is termed anesthetic or pharmacologic preconditioning. Ischemic preconditioning for 7- to 10-min occlusions, such as those required for OPCAB and MIDCAB, probably does not provide the same benefit as one might see with longer periods of occlusion, but this technique is employed by some surgeons.
d. The proximal anastomosis of a vein graft can be performed first in order to allow immediate perfusion once the distal anastomosis is completed.
e. Regional hypothermia techniques have been described for use during coronary occlusion.
f. Preoperative insertion of an intra-aortic balloon pump (IABP) has been used for patients with reduced ventricular function requiring multivessel OPCAB.
D. Patient selection: High risk versus low risk
1. Early reports of OPCAB often described single-vessel or double-vessel bypass performed on low-risk patients. This was promoted as permitting early recovery and discharge.
2. OPCAB is now promoted for multivessel bypass in patients with risk factors for adverse outcomes. Elderly patients at risk for stroke, patients with severe lung disease, or patients with severe vascular disease and/or renal dysfunction are often selected. As mentioned earlier, scientific studies have not yet demonstrated reduced adverse outcomes with OPCAB in these populations.
3. Zenati et al. [19] and others have described combining MIDCAB (i.e., IMA to LAD) with angioplasty/stent to other vessels in high-risk patients.
4. As mentioned earlier, a small number of surgeons attempt to perform virtually all CABG procedures as OPCAB regardless of preoperative risk status.
E. Anesthetic management
1. Preoperative assessment
a. The cardiac catheterization report should be reviewed and the procedure discussed with the surgeon, including the planned sequence of bypass grafts and the potential use of specific adjuncts (e.g., shunts or perfusion-assisted direct coronary artery bypass grafting, or PADCAB). This allows the anesthesiologist to predict the effect of each coronary occlusion, which requires knowledge of the coronary anatomy and its usual nomenclature (Fig. 13.2).
Figure 13.2 Coronary anatomy. The main branches from the circumflex artery (CX) are named “marginal” or “obtuse marginal” vessels. D1, first diagonal; D2, second diagonal; LAD, left anterior descending artery; LM, left main; PDA, posterior descending artery; RCA, right coronary artery; LVBr, LV branch; Ra, Ramus intermedius (<40% of individuals).

b. The vessel, location, and degree of stenosis determine the functional response to intraoperative coronary occlusion. Even with a proximal stenosis, an important vessel (e.g., LAD) may supply adequate resting flow to a large area of myocardium. Acute loss of flow to this large area (with surgical occlusion) may cause ventricular failure. A stenosis further down the vessel may be less important for overall ventricular performance.
c. High-grade stenosis (e.g., 90%) is likely to be associated with some collateral blood flow from adjacent regions, as flow through the stenosis may be inadequate even at rest. A 10-min occlusion of such a vessel may have surprisingly little effect on regional function and hemodynamics because of the collateral flow. A lesser degree of stenosis (e.g., 75% to 80%) may not affect resting flow, hence there may be little or no collateral blood flow. Occlusion of such a vessel may cause severe myocardial dysfunction in the distribution of the vessel.
d. If incisions other than sternotomy are to be employed to access specific coronary regions, positioning of the arms and the body, the potential need for one lung anesthesia, and sites for vascular access need to be discuss. Some surgeons prefer one-lung anesthesia even for a median sternotomy approach to OPCAB.
2. Measures to avoid hypothermia
a. Unlike on-pump CABG, it is difficult to restore heat to a hypothermic OPCAB patient. In order to maintain hemostasis and facilitate early recovery, prevention of heat loss needs to be planned before the patient enters the room.
b. While in the preoperative area, the patient should be kept warm with blankets.
c. The operating room should be warmed to the greatest degree tolerated by the operating team (e.g., 75°F or higher). The temperature can be reduced once warming devices have been placed and the patient is fully draped.
d. The period and degree to which the patient remains uncovered for preoperative procedures (e.g., urinary catheter placement) and surgical skin preparation and draping should be minimized. This requires vigilance on the part of the anesthesiology team and frequent reminders to the surgical team.
e. Various adjuncts to preserve heat include heated mattress cover or insert; forced-air warming blankets, including sterile “lower body” blankets placed after vein harvesting; and circumferential heating tubes. A more expensive and possibly more effective option is the use of disposable surface-gel heating devices [20].
f. Fluid warmers should be used at least for the principal intravenous volume infusion “line,” if not for all intravenous lines other than those used for intravenous drug infusions.
g. Low fresh-gas flows and circle/CO2 reabsorption circuits will help prevent heat loss.
3. Monitoring (Table 13.1)
Table 13.1 Monitoring approaches for OPCAB and MIDCAB
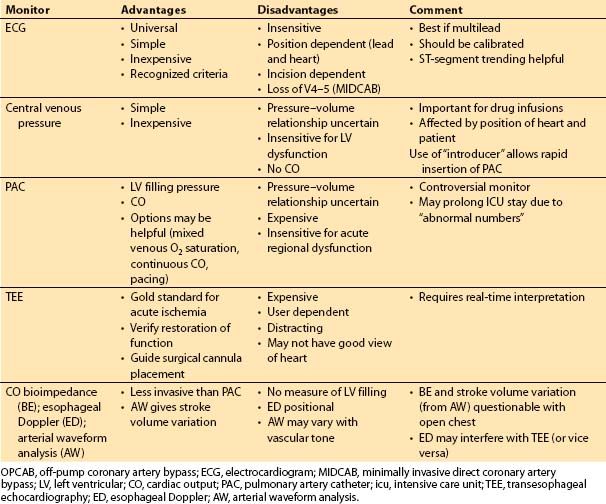
a. Preoperative assessment of ventricular function
(1) Preoperative LV function is a major determinant of the need for extensive monitoring. Patients with normal or near-normal LV function are less likely to need diagnosis and therapy guided by invasive monitoring.
(2) A patient with an elevated LV end-diastolic pressure (at cardiac catheterization) may have a “stiff” ventricle, or diastolic dysfunction. This commonly results from hypertrophy or ischemia. Filling pressures obtained intraoperatively must be interpreted in this context (i.e., the filling pressure may overestimate LV preload). Volumetric assessment of preload (by TEE) can be valuable in this situation.
(3) Patients with poor ventricular function may tolerate coronary occlusions poorly. Appropriate responses may be best guided by monitors of cardiac output (CO) and filling pressures, or TEE [21,22].
(4) Repeated occlusions in multiple regions of the myocardium (i.e., for multivessel OPCAB) are likely to result in a cumulative detrimental effect on hemodynamics. There may be a period of myocardial dysfunction requiring inotropic support even in patients with good underlying LV function. The combination of reduced ventricular function and the need for multiple bypass grafts is likely to result in a need for inotropic and/or vasopressor infusions guided by monitoring with a pulmonary artery catheter (PAC) and/or TEE.
(5) Preoperative placement of a PAC introducer, but with an obturator of some kind or a single- or double-lumen central venous catheter placed through it rather than a PAC may be a reasonable first approach in most patients. This avoids the use of the PAC in uncomplicated patients while allowing for rapid PAC placement should this be desired any time in the perioperative period.
b. Specific monitors
(1) Lead V5 of the electrocardiogram (ECG) detects 75% of the ischemia found on all 12 leads. This lead should be monitored in all patients undergoing OPCAB or MIDCAB, as permitted by the surgical incision. Lead II gives clear P waves, but adds little to the sensitivity of ischemia detection.
(2) The PAC is variably useful during OPCAB. For single- or double-vessel bypass in patients with preserved LV function, there can be little justification for this monitor [23]. The worse the ventricular function and the greater the number of planned bypass grafts, the more likely it is that information from the PAC will be useful.
(3) Continuous CO from the PAC or other devices and continuous mixed venous oximetry may provide incremental benefit in assessing the adequacy of cardiac function. Use of these devices is often institution-specific or even surgeon/ anesthesiologist-specific.
(4) Monitoring with TEE can provide detailed information about the effects of coronary occlusion and recovery, and it provides the earliest, most specific information during acute deterioration and interventions. Acute ventricular dilatation and mitral regurgitation may occur when a large region of the myocardium becomes ischemic, and this is detected immediately with TEE. In addition, distortion of the mitral annulus due to abnormal positioning may cause mitral regurgitation [24]. Obtaining adequate images may be distracting to clinical care. With the heart in an unusual position, images may be difficult or impossible to obtain. A reversible wall-motion abnormality that resolves with restoration of flow is reassuring; however, this does not guarantee a good quality graft or anastomosis.
6
(5) Normal carbon dioxide (CO2) elimination requires adequate CO. If ventilation is constant, an acute decline in CO will cause an acute decrease in end-tidal CO2 concentration.
c. Monitoring for specific procedures
(1) For MIDCAB or other reduced-access procedures, provision must be made for transcutaneous defibrillation and pacing. An important consideration is the requirement to reinflate the lungs for defibrillation during closed-chest surgery to provide tissue (rather than air) for the current to traverse [25].
(2) For port-access surgery (Heartport or related procedures), TEE is required to guide and monitor cannula placement and function.
4. Anesthetic technique
7
a. Early recovery is usually desired. Extubation immediately or shortly after surgery should be the goal.
b. A vapor-based anesthetic technique facilitates early recovery. Keys to prevention of delayed awakening are as follows: Minimizing the dose of benzodiazepine; use of modest doses of opioids; and avoiding residual paralysis at the end of surgery. Some clinicians use very short-acting opioids such as remifentanil to facilitate early extubation, but this approach requires awareness of the need for effective longer-lasting analgesia at the time of extubation and thereafter. Use of bispectral index (BIS) monitoring can help guide administration of hypnotic agents.
c. Transfer of the intubated yet awakening patient to the intensive care unit (ICU), and early ICU care are facilitated by use of short-acting sedative drugs such as propofol or dexmedetomidine.
d. Thoracic epidural or lumbar spinal anesthetic and analgesic techniques have been promoted by some as suitable adjuncts to off-pump approaches. There are reports of OPCAB procedures done without general anesthesia. Most centers are reluctant to risk major neuraxial techniques immediately before full heparinization for CPB. Use of such techniques is unlikely to shorten postoperative length of stay and has not been shown to provide a measurable benefit.
e. For MIDCAB (thoracotomy), postoperative epidural analgesia [26], paravertebral block, or intercostal blockade may be useful for pain control.
5. Anticipation and management of ischemia
a. Knowledge of the coronary anatomy and surgical plan is essential. This allows appropriate timing of pharmacologic and other interventions before ischemia is induced. Use of isoflurane (or other volatile inhalational agent) anesthesia can provide pharmacologic “preconditioning,” as mentioned earlier. Avoidance of hemodynamic alterations associated with ischemia such as tachycardia (especially in the presence of hypotension) must be avoided. Intravenous β-adrenergic blockade may be beneficial; however, this must be balanced with the possibility of impaired myocardial performance during coronary occlusion.
b. Maintenance of adequate coronary artery perfusion pressure is of great importance in allowing collateral blood flow to ischemic regions. Volume loading and appropriate positioning (see following), alteration of the depth of anesthesia, and/or use of α-adrenergic agonists may all be indicated.
c. Prophylactic nitrate infusions may interfere with preload (see later).
d. Early experience without modern stabilizers suggested that bradycardia (to reduce motion) would aid the surgeon. This is no longer an issue. Grafting to the RCA territory (supplying the sinus and AV nodes) can be associated with bradycardia. Thus, although β-adrenergic blockade may be useful to prevent or treat tachycardia, epicardial pacing may be required for ischemia-induced bradycardia.
e. Anecdotally, patients with compromised ventricular function undergoing multivessel procedures may benefit from “prophylactic” administration of an inotropic medication.
3
6. Intravascular volume loading
a. Positioning of the heart may kink or partially obstruct venous return and/or compress the RV. Intravascular volume loading and head-down (Trendelenburg) position can help reduce this effect (Fig. 13.3) [27]. Close observation of the heart, filling pressures, and blood pressure to provide feedback to the surgeon is essential.
Figure 13.3 Relative changes in hemodynamic parameters during vertical displacement of the beating porcine heart by the Medtronic Octopus tissue stabilizer and the effect of head-down tilt. BASE, pericardial control position; Cx, circumflex coronary artery; DIS, displacement of the heart by the Octopus; DIS + TREND, Trendelenburg maneuver (20-degree head-down tilt while the heart remains retracted 90 degrees); LAD, left anterior descending artery; RCA, right coronary artery; x = mean arterial pressure. Statistical comparison with control values: *p < 0.05; **p < 0.01; #p < 0.001; ^p = 0.046 versus combined relative value of LAD and RCA flows. (From Grundeman PF, Borst C, van Herwaarden JA, et al. Vertical displacement of the beating heart by the Octopus tissue stabilizer: Influence on coronary flow. Ann Thorac Surg. 1998;65:1348–1352, with permission.)

b. Intravenous vasodilators (e.g., nitrates) can exacerbate reductions in cardiac filling. More commonly, intravenous vasoconstrictors (phenylephrine, norepinephrine) will be required during abnormal cardiac positions.
7. Surgery-anesthesiology interaction. With all the above considerations, it should be clear that there must be excellent communication between the surgeon and the anesthesiologist for OPCAB or MIDCAB. Anticipation and planning for problems allow the anesthesiologist to intervene in a timely manner. The surgeon must say in advance what he is planning to do. Similarly, changes in cardiac performance and the need for intervention must be continuously communicated to the surgeon. The anesthesiologist must observe the surgical field, watching the procedure as well as the position, size, and function of the heart. An observant, communicative team with basic monitoring (ECG, blood pressure, and central venous pressure) is likely to produce better results than a team that communicates poorly, but uses extensive monitoring.
F. Anticoagulation
1. Heparin management
a. Heparin anticoagulation protocols are institution-specific. Similar to on-pump surgery, there are few data to recommend targeting specific activated clotting time (ACT) values.
b. Some surgeons request full heparinization similar to on-pump procedures (i.e., ACT target >400 s); others request lower doses of heparin such as would be used for noncardiac vascular procedures (ACT target typically >200 s), or something in between. Outcomes appear to be equivalent using either approach, which suggests that ACT targets as high as those used for CPB are unnecessary.
2. Protamine reversal
a. Extracorporeal circulation induces a postoperative multifactorial defect in coagulation that may reduce early graft thrombosis. When coagulation is reversed after OPCAB or MIDCAB, no such hypocoagulable state exists; indeed, there is evidence that the coagulation system is activated by the stress of surgery, similar to what has been showed for other major procedures [28].
b. In order to gradually return the coagulation to normal leaving perhaps a little residual heparin effect, reversal may be achieved with incremental doses of protamine. If “full” heparinization has been employed, administration of 50 mg of protamine may bring the ACT down to near 200 s, after which small increments (10 to 25 mg) can be given to achieve an ACT that is about 25% to 50% above control (i.e., 150 to 180 s).
c. If the patient is clinically bleeding with an elevated ACT, then heparin should be reversed completely. Even in the absence of clinical bleeding, some cardiac surgeons prefer complete reversal immediately after completion of the grafts.
d. Prolonged OPCAB procedures may be associated with extensive blood loss and therefore facilitated by the use of cell-saver devices (i.e., washing of salvaged blood so it is free of coagulation proteins and platelets). Over time, this may induce a dilutional coagulopathy similar to what is often seen after CPB.
3. Antiplatelet therapy
a. Thrombosis at the site of vascular anastomoses is initiated by platelet aggregation and adhesion. Similar to strategies that are used in angioplasty/stent procedures, antiplatelet therapy may help reduce early graft thrombosis in CABG, whether done with or without CPB.
b. A common practice is to administer a dose of aspirin preoperatively. This can be achieved with a suppository if the patient is already anesthetized.
c. In on-pump CABG, administration of aspirin within 4 h after the procedure reduces graft thrombosis. This strategy should also be applied to OPCAB and MIDCAB.
d. There are no published data about the use of newer antiplatelet drugs in this setting. As with all such therapies (including aspirin), the concern for bleeding must be balanced with the desire to prevent graft thrombosis.
4. Antifibrinolytic therapy. Use of lysine analogs to inhibit fibrinolysis has become common practice with on-pump CABG, as they have been shown to reduce perioperative blood loss. Recent investigations now support the use of these agents during OPCAB as well [29].
G. Recovery
1. Extubation in the operating room
a. For uncomplicated procedures, recovery from OPCAB or MIDCAB can be rapid without the requirement for postoperative ventilation or sedation.
b. Normothermia, hemostasis, and hemodynamic stability must be assured.
c. Residual anesthesia and paralysis from long-acting agents (e.g., pancuronium, large doses of morphine) must be avoided.
d. Extra time spent in the operating room to achieve extubation may be more costly than a few hours of postoperative ventilation and sedation.
2. ICU management
a. For most patients, early postoperative management can employ the “fast track” technique where mechanical ventilation is withdrawn within a few hours of surgery, and patients are extubated and possibly mobilized at the bedside late in the day or during the evening of surgery.
b. ICU stay is driven by institutional practice, but for patients having straightforward, uncomplicated procedures, there may be no need for more than a few hours in a high-intensity nursing area (i.e., postanesthetic care unit or ICU).
c. If length of stay is reduced, cost will almost certainly be reduced. If there is no significant reduction in stay, the cost of specialized retractor systems may exceed the cost of the disposables required for CPB.
d. Some surgeons passionately believe that OPCAB is better for their patients; perhaps with time and additional randomized trials, the hoped-for reductions in neurologic events, renal dysfunction, and other adverse outcomes will become apparent.
II. Minimally invasive valve surgery (MIVS)
A. Introduction
The Society of Thoracic Surgeons National Database defines minimally invasive surgery as “any procedure that has not been performed with a full sternotomy and cardiopulmonary bypass support. All other procedures, on- or off-pump with a small incision or off-pump with a full sternotomy are also considered minimally invasive” [30]. Similar to MIDCAB, the premise of MIVS is that “smaller is better” for valve surgery as well. A partial sternotomy or small thoracotomy with port incisions may achieve some benefits when compared to standard median sternotomy. Similar to OPCAB, alternative approaches were explored in the late 1990s, with the first publication in 1998. Proposed [31,32] but unproved advantages to these approaches include the following:
1. Reduced hospital length of stay and costs
2. Quicker return to full activity
3. Less atrial fibrillation (26% vs. 38% in one report [33])
4. Less blood transfusion
5. Same results (mortality, valve function)
6. Less pain
7. Earlier ambulation
In addition, the surgical opinion is that reoperation should be easier after MIVS, as the pericardium is not opened over the RV outflow tract. These proposed benefits may be observed with specific surgeons in specific centers; however, there have been no rigorous or randomized studies. The limited data that exist suggest that acute postoperative pulmonary function impairment is not improved by the use of the limited incision. Minimally invasive reoperative aortic valve surgery is a new and successful technique, especially in patients who have had previous cardiac operations using a full sternotomy (e.g., prior CABG). This surgical approach does not disturb the vein grafts or the patent IMAs [34].
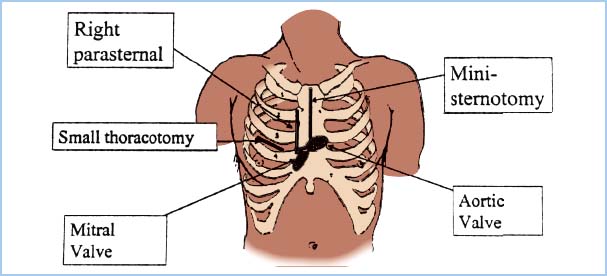
B. Surgical approaches (Fig. 13.4)
8
1. Port-access (Heartport): This approach involves direct surgical visualization and operation through small openings (ports) and a small right horizontal thoracotomy incision for access to the mitral valve or atrial septum. In order to avoid sternotomy, the port-access system uses alternative access sites and cannulae. The aorta is cannulated through a long femoral arterial catheter or a shorter transthoracic aortic catheter. These devices are advanced to the ascending aorta and include an “endo-aortic clamp” or inflatable balloon to achieve aortic occlusion (“cross-clamp”) from within. They include a cardioplegia administration port. Venous cannulation is achieved with a long femoral venous catheter, supplemented as needed with a pulmonary artery vent. A coronary sinus catheter is used to administer cardioplegia. Placement of these catheters can be time-consuming and requires imaging with fluoroscopy and/or TEE. A limited number of institutions still use this approach to MIVS. Some reports have raised concerns about device-related aortic dissections as well as endoaortic balloon rupture and dislocation [35].
2. Video-assisted port-assisted (using the port-access cannulae and small incisions with video equipment to visualize and perform valve surgery). This is currently performed in a small number of institutions worldwide.
3. Robotic (see below). This is an evolving technique, particularly for mitral valve repair, with excellent results being reported.
4. Direct-access (small incision—many types: Anterolateral mini-thoracotomy, partial upper or lower sternotomy, right parasternal incision, and others). The right parasternal approach is preferred by some surgeons (especially for aortic valve access) because there is no sternal disruption and it is cosmetically pleasing. The avoidance of sternotomy bleeding into the pericardial sac with associated fibrinogen depletion may result in less perioperative bleeding and less pain that is easier to control, although the need to divide two or more costochondral cartilages with the parasternal approach does induce considerable postoperative pain. As mentioned earlier, avoidance of opening the pericardium over the RV outflow tract may make future cardiac reoperation easier and safer. One problem with this approach is the sacrifice of the right IMA.
5. The current standard approach for aortic valve surgery using a minimally invasive approach is an “inverse L” shaped partial sternotomy extending to the third or fourth right intercostal space. In order to bring the entire heart more anteriorly, three to four retention stitches are passed through the pericardial rim and fixed to the skin incision. This may be associated with compression of the right atrium and a decrease in the venous return to the heart. The arterial cannula for CPB is placed in the ascending aorta. Venous return is established by direct cannulation of the right atrial appendage or by percutaneous femoral vein cannulation of the right atrium. With the latter cannulation technique, often a two-stage cannula is used with the distal tip placed at the junction of the SVC and RA as confirmed by TEE (bicaval view) [36].
6. Minimally invasive mitral valve surgery can use a right anterolateral minithoracotomy for which the patient is positioned supine with slight elevation of the right chest (often using a folded blanket) and extension of the right arm. Optimal visualization the heart requires deflation of the right lung, which is achieved most often by using a double-lumen endobronchial tube. CPB is established via the femoral vessels. The superior vena cava may also be cannulated percutaneously via the right internal jugular vein (IJV). A percutaneous transthoracic aortic cross-clamp is placed through a separate stab incision in the right posterior axillary line [35], and the mitral valve is accessed through the left atrium. Another approach is a right parasternal incision with mitral valve exposure via the right atrium and interatrial septum.
7. Reduced-size skin/soft tissue incision compared with full median sternotomy can give a more cosmetically pleasing result.
Figure 13.4 Incisions for MIVS. The most common approach is the “mini” sternotomy, which extends from the sternal notch part way to the xiphisternum but is diverted to the right at the level of the third or fourth interspace (for the aortic valve), leaving the lower sternum intact. The mitral valve can be accessed through the small right thoracotomy. (From Clements F, Glower DD. Minimally invasive valve surgery. In: Clements F, Shanewise J, eds. Minimally Invasive Cardiac and Vascular Surgical Techniques. Society of Cardiovascular Anesthesiologists monograph. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:30.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








