TECHNOLOGY ASSISTED CHILDREN
JOEL A. FEIN, MD, MPH, KATHLEEN M. CRONAN, MD, AND JILL C. POSNER, MD, MSCE, MSEd
Nearly one-fourth of the visits to a pediatric emergency department (ED) are for complaints associated with chronic illness. Many children with chronic illness have indwelling medical devices, such as cerebrospinal fluid (CSF) shunts, venous catheters, and gastrostomy tubes (G-tubes). Medical technology has enabled these children, who in the past would have required specialized inpatient or intensive care, to thrive at home. Emergency physicians must be able to diagnose and treat the common problems associated with these devices and recognize when it is appropriate to consult other specialists familiar with these children.
Devices most commonly found in the pediatric population include CSF shunts, tracheostomy tubes, venous catheters, and percutaneous gastrointestinal (GI), and urologic catheters. This chapter familiarizes the emergency clinician with the equipment and with the clinical manifestations and management of the problems related to these apparatuses.
APPROACH TO THE CARE OF THE TECHNOLOGY-ASSISTED CHILD
The evaluation of the technology-assisted child who visits the ED may, at times, seem overwhelming. These children are often assisted by several pieces of equipment, the history can be difficult to obtain because of its inherent complexity, and a thorough physical examination may be impeded by the technology. When a common illness is superimposed on a chronic condition, the illness may appear more complex, misleading the examiner. In addition, the ED visit may have been prompted by multiple reasons. The more involved the equipment and problems, the more challenging the situation becomes.
When a technology-assisted child arrives in the ED, early contact with the primary care provider may be helpful. The primary care provider may be able to offer suggestions about the management of the child, potentially avoiding unnecessary tests and admission. In many situations, a home health nurse may accompany the patient to the ED and can be a valuable source of information.
The American College of Emergency Physicians and the American Academy of Pediatrics have provided an Emergency Information Form for children with special health care needs, available at www.aap.org/advocacy/epcparent.htm or http://www.acep.org/patients.aspx?id=26276. This form can be accessed at the time of an ED visit for patients who have subscribed to the service. A MedicAlert bracelet provides a patient identification number that enables procurement of information about the patient. By accessing the MedicAlert hotline, relevant medical information about the patient can be faxed rapidly to the ED for immediate use. This can help improve the accuracy of the history and improve the quality of care administered.
When caring for the technology-assisted child, several important principles emerge that should be used in the acute care setting (Table 143.1).
First, common things are common; common pediatric illnesses may afflict these children. This point is always important to remember when evaluating a seemingly complicated child who presents with the routine signs and symptoms characteristic of typical childhood diseases. For example, a child with a CSF shunt may have vomiting caused by gastroenteritis.
Second, the presence of indwelling devices predisposes the patient to infection. When a child presents with symptoms associated with a specific piece of equipment, the clinician must be suspicious of infection of that equipment. For example, if a child with a tracheostomy presents with fever, cough, and increasing secretions, it is crucial to evaluate for the possibility of tracheitis. At the same time, the equipment has a tendency to become colonized with commensal organisms. Therefore, all bacterial growth does not indicate acute infection and other sources of infection should be considered.
Above all, families should be relied on for important information because they often have become knowledgeable of specific illnesses and equipment. Parents are sensitive to subtle changes in their children. Families are experts and should play an integral role in the evaluation, management, and ultimate disposition of their child in the ED setting.
Children with chronic illnesses have a higher likelihood of being admitted to the hospital, resulting in longer lengths of stay in the ED. The practitioner should realize that the families of technology-assisted children often have sufficient equipment and trained personnel available in the home setting to care for an exacerbation of a chronic problem or an unrelated acute problem. For example, family members whose child has a chronic respiratory illness often have supplemental oxygen in the home and are facile with its use. Knowing that families of technology-assisted children are compliant and likely to return to the ED if their child’s degree of illness exceeds the capabilities of the home care is reassuring. Thus, the practitioner should consider altering the usual criteria for admission in this specific population. On the other hand, technology-dependent children may show more subtle signs of illness and can deteriorate rapidly.
Having a technology-assisted child in the home creates a stressful situation for family members and other caregivers. A visit to the ED for an acute problem exacerbates this level of stress. These families may be more likely to question the diagnostic tests and therapies offered during the evaluation of their child because of their level of medical knowledge, as well as the constant illness-related anxiety that intrudes upon their lives. The ED visit is more effective if the practitioner recognizes the psychosocial issues associated with this population of patients.
TABLE 143.1
APPROACH TO THE TECHNOLOGY-DEPENDENT CHILD IN THE EMERGENCY DEPARTMENT

TRACHEOSTOMY CARE
Background
Advances in neonatology and pediatric critical care medicine have enabled children to survive the complications of premature birth, congenital anomalies, and severe life-threatening illnesses. As home care has become more widely recognized as an alternative to prolonged and costly hospitalization, the number of children managed at home with tracheostomies and mechanical ventilation has increased dramatically. Consequently, the number of such children seeking care in the ED has also increased. To approach these situations calmly and systematically, the emergency physician should (1) appreciate the physiologic differences in a patient with chronic respiratory insufficiency (CRI), (2) be familiar with the equipment used in the care, and (3) understand the commonly encountered complications and their management.
Pathophysiology
In healthy people, respiration is maintained via a complex mechanism involving the alveolocapillary network, the diaphragm and intercostal musculature, and the central respiratory centers in the brainstem. Respiratory compromise results when one or more components of this mechanism are affected by disease. Chronic respiratory support may be a part of the management plan for children with diverse disease processes, including neurologic and neuromuscular disorders, central hypoventilation syndromes, obstructive apnea, congenital facial and airway anomalies, and others. Processes such as bronchopulmonary dysplasia once accounted for the majority of CRI; however, recent epidemiologic studies have demonstrated a shift in the proportion of CRI from chronic lung disease to neurologic and neuromuscular disorders.
Equipment
The complexity of the many tubes and attachments extending from the patient’s airway can be overwhelming, especially in the emergent situation. Familiarity with the equipment used in caring for a patient with a tracheostomy ensures the emergency physician’s adept management of these situations. Starting from the patient’s neck, each piece of equipment can be easily identified (Fig. 143.1).

FIGURE 143.1 Tracheostomy parts.
Tracheostomy Tubes
Modern tracheostomy tubes are made of polyvinylchloride, a soft substance that conforms to the shape of the trachea, but is rigid enough to avoid collapse. Unlike their metal predecessors, they have little tissue reactivity, causing less tracheal wall irritation. Several manufacturers package sterile tracheostomy tubes for one-time use. Intensivists directing the long-term airway management of their patients may prefer one manufacturer to another, but the emergency physician does not need to know the minor differences among the products. The emergency physician should, however, know what types of tracheostomy tubes are stocked by the ED’s facility and how to convert from the patient’s brand and size to an available tube with suitable dimensions.
Three dimensions determine the size of a tracheostomy tube: the inner diameter, the outer diameter, and the length. The inner diameter refers to the same measurement used in describing the size of an endotracheal tube, ranging from 2.5 to 10 mm. This measurement is generally imprinted on the flanges of the tracheostomy tube and is standardized among manufacturers. The outer diameter and length are often not identified on the tube and can vary considerably among manufacturers. The chart in Table 143.2 lists the dimensions of various tubes. When a tracheostomy tube change is indicated and an identical replacement is not available, this chart can be used in selecting the appropriate size tube of an available type.
A tracheostomy tube may be cuffed or uncuffed. An infant or young child may have a cuffed tracheostomy tube, especially if he or she has an airway anomaly or has developed tracheomegaly. Checking for the presence of a cuff is important because the cuff must be deflated before removing the tube.
Some tracheostomy tubes are fenestrated. The hole in the posterior aspect of the tube facilitates retrograde movement of air through the larynx, allowing vocalization. In addition, some tracheostomy tubes have an inner cannula that is positioned within the lumen of the tracheostomy tube (i.e., the outer cannula) so that it can be removed for cleaning while the airway is maintained by the outer cannula. Importantly, the proximal portion of the inner cannula is required to connect the tracheostomy to the manual resuscitator bag; therefore, the inner cannula must be in place when bag-valve ventilation is performed (Fig. 143.2).
TABLE 143.2
TRACHEOSTOMY TUBE DIMENSIONS
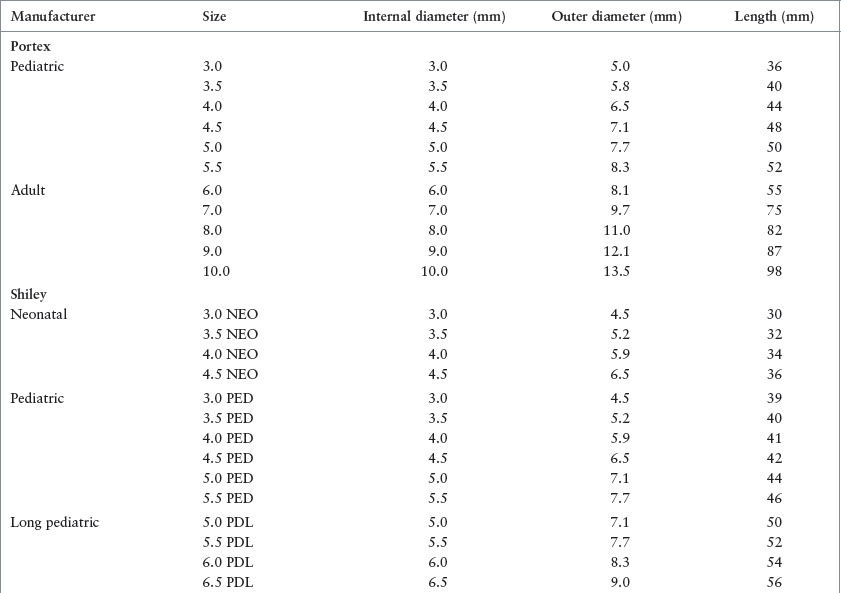


FIGURE 143.2 Dual cannula tracheostomy tube.
Swivel
A swivel is often attached to the end of the tracheostomy tube. Some unique characteristics of children make the swivel particularly useful. First, children have a natural inclination to move and explore. The swivel device accommodates movement in the ventilator-assisted child, so traction is not placed on the ventilator tubing or on the tracheostomy tube. Second, the short neck and bulky soft tissues of young children can obstruct the tracheostomy tube opening. The swivel provides additional length, so the tube opening extends beyond the soft tissues of the neck.
Heat–Moisture Exchanger
Air inspired directly into the trachea through a tracheostomy tube bypasses the important warming and humidification mechanisms provided by the natural upper airway. Therefore, a humidification system is an important component of the equipment used in a patient with a tracheostomy. A home ventilator setup includes a stationary humidification system that is used when the child is connected to the circuit. Similarly, a heat–moisture exchanger is attached to the end of the tracheostomy tube in patients who do not require the ventilator. The device is composed of a hydrophilic material that captures the patient’s own heat and humidity on exhalation so that it can be inspired on inhalation.
Clinical Findings/Management
The approach to the ill patient with an artificial airway is the same as that for any patient who comes to the ED. The initial evaluation consists of an assessment of the patient’s ABCDs (airway, breathing, circulation, and disability), with particular attention to the airway and breathing. An emergency physician who knows how to anticipate common problems and to recognize them early is able to institute appropriate therapy without delay.
Obstruction and Decannulation
The most life-threatening complication in a patient with an artificial airway is cannula obstruction or dislodgment. Younger children are more likely to experience accidental decannulation because of the short length of the trachea and tracheostomy tube. Some infant tubes are as short as 3 to 4 cm. In addition, the small lumen is more easily occluded by a mucous plug or by an accumulation of secretions. Infants with less-developed intercostal muscles and children with neuromuscular disorders may be unable to generate an adequate cough to keep the airway clear of debris.
The presentation is similar to that of other children with airway obstruction. The child may appear distressed with tachypnea, cyanosis, accessory muscle use, and/or nasal flaring. Alternatively, the child may be lethargic or obtunded as a result of prolonged respiratory effort or an elevated carbon dioxide level.
Any child with an artificial airway and respiratory distress is assumed to have an obstruction. The patient should be placed immediately on high-flow humidified oxygen. The physician should determine whether the tracheostomy tube appears to be in place, recognizing that a tube in the stoma does not necessarily indicate a tube in the trachea. If a cannula change was attempted before the child’s arrival in the ED, a false passage into the paratracheal soft tissues may have occurred. Auscultation for the presence and symmetry of bilateral breath sounds should be performed and the quality of the patient’s respiratory effort should be assessed. Immediate suctioning is appropriate in an attempt to assess tube patency and to clear the airway of secretions.
The physician should not hesitate to change the cannula. Suctioning alone may not clear an obstruction caused by thick secretions. All the necessary equipment for the change should be present, including a replacement tracheostomy tube, an endotracheal tube one-half size smaller, and a bag-valve-mask ventilation circuit with oxygen flow, scissors, and tracheostomy ties. The change is best accomplished with the participation of two people: one secures the patient and removes the old tube, whereas the other inserts the new tube. Remember to deflate the cuff prior to removal, if one exists.
Infection
Bacterial colonization of the trachea usually occurs in a child with a tracheostomy. Common colonizing organisms include gram-positive cocci (Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pneumoniae, α- and β-hemolytic streptococci), gram-negative bacilli (Klebsiella, Pseudomonas, Escherichia coli, Serratia marcescens, Haemophilus influenzae), and anaerobes (Peptostreptococcus, Bacteroides). These same organisms can become pathogenic, causing tracheitis or pneumonia.
A peristomal cellulitis can result from infection with skin flora. Good tracheostomy care and regular cleaning with dilute hydrogen peroxide solution can prevent most peristomal infections. Similarly, inadequate padding of the neck area beneath the tracheostomy ties can result in a contact or monilial dermatitis. Differentiating between bacterial colonization of the trachea and clinical infection can be difficult. The physician should elicit a history of any changes in the quantity, thickness, or odor of the tracheal secretions, and any systemic signs of infection or respiratory distress. Along with physical examination, there should be a determination of oxygenation by pulse oximetry. A Gram stain and bacterial culture, and a rapid viral detection assay of the tracheal secretions, may be helpful in determining the presence and cause of an infection. Leukocytosis in the tracheal secretions and a predominant organism by Gram stain may be suggestive of bacterial tracheitis; radiographic evidence of a new infiltrate indicates pneumonia.
If the child appears well and follow-up can be ensured, outpatient antibiotic therapy may be appropriate. For children with increased oxygen or ventilatory requirements, hospitalization should be considered for intravenous (IV) antibiotic therapy, aggressive pulmonary toilet, and close monitoring.
Erythema of the peristomal skin is usually caused by irritation and should be managed by increasing the frequency of the tracheostomy care at home. The additional findings of warmth, tenderness, purulent drainage, or fever may suggest the presence of a peristomal cellulitis. Depending on its severity, this condition should be treated with oral or IV antibiotics.
The skin of the neck under the ties securing the tracheostomy tube can also become inflamed. Generally, this situation can be treated by increasing the amount of padding and by keeping the area dry. An erythematous rash with satellite lesions classic for a monilial dermatitis should be treated with topical antifungal creams.
Asthma
The incidence of asthma in children with chronic lung disease has increased. Many children are maintained at home on inhaled β-agonists and inhaled steroids therapy. The usual viral and environmental triggers, such as dust, pets, and smoke, precipitate exacerbations of asthma in these children.
The presentation is similar to that of other asthmatic patients, with varying amounts of respiratory distress, wheezing, and hypoxemia. As previously mentioned, the physician must consider the possibility of cannula obstruction or dislodgment in all cases. Treatment with oxygen, bronchodilators, and steroids should be initiated promptly. Emergency clinicians should recognize, however, that children with chronic lung disease have less pulmonary reserve. Chest radiography and arterial blood gas analysis should be performed as clinically indicated. Increased ventilatory support or continuous positive airway pressure may be required to overcome fatigue and atelectasis.
Bleeding and Granuloma
The tracheal mucosa located adjacent to the stoma, the cuff, and the distal tip of the tracheostomy tube is prone to bleeding and granuloma formation. The most common reason for bleeding is inadequate humidification causing drying and friability of the tracheal mucosa. Infection or granuloma formation can also result in small amounts of bleeding. Large amounts of blood coming from the tracheostomy tube opening can signify erosion of the tube into the brachiocephalic artery. The incidence of tracheoarterial fistula formation is rare (approximately 0.7%) but commonly results in death due to massive hemoptysis and blood loss. The risk for development of this life-threatening complication is highest during the postoperative period (i.e., within 4 weeks of tube placement).
Small amounts of bleeding from the tracheal stoma usually resolve with increased humidification of the inspired air. The persistence of minor bleeding might indicate an intratracheal granuloma, which should be evaluated by direct visualization. This procedure is best performed by an otorhinolaryngologist.
A large amount of bleeding is a surgical emergency. IV access should be obtained immediately and volume replacement should be initiated. The tracheostomy tube should not be removed because it may be the best way to ensure an airway. Frequent suctioning aids in preventing aspiration. If the site of bleeding can be identified, direct pressure should be applied to the area. Overinflating the cuff may tamponade a bleeding vessel and provide a temporary treatment until it can be ligated.
Peristomal granulomas can usually be treated with topical antibiotics. In refractory cases, cauterization with silver nitrate is indicated.
CEREBROSPINAL FLUID SHUNTS
Background
CSF shunt placement is the most common neurosurgical procedure performed in children. More than 4,400 CSF shunts were placed in 2003; CSF shunt–related problems accounted for almost 15,000 hospital admissions and almost $300 million were charged for shunt malfunctions. CSF shunts are placed to divert CSF from the brain to another area of the body, most commonly the peritoneal cavity. The clinician evaluating a child with a CSF shunt should be aware of associated complications such as infection, obstruction, and overdrainage, because certain complications can be disastrous if unrecognized and untreated. However, children with CSF shunts may often exhibit symptoms of their chronic illnesses that are unrelated to shunt malfunction.
Pathophysiology
CSF is an ultrafiltrate of plasma produced at a rate of 500 mL per day in a 70-kg adult and proportionally less in children and infants. The fluid is mainly produced by the choroid plexus and various extrachoroidal sites within the brain. CSF travels from the lateral ventricles into the third ventricle through the foramen of Monro and then again through the aqueduct of Sylvius to the fourth ventricle. The CSF then enters the subarachnoid space via the foramina of Luschka and Magendie and travels through the brain and spinal canal. CSF is reabsorbed and enters the venous system through the “one-way valves” of arachnoid villi that penetrate the dura.
Hydrocephalus can result from oversecretion, impaired absorption, or blockage of CSF pathways. Oversecretion can occur in some choroid plexus tumors. Impaired absorption can occur as a result of increased CSF protein, often a result of perinatal hemorrhage or meningitis or less commonly etiologies such as subarachnoid hemorrhage, or Guillain–Barré syndrome. Severe congestive heart failure or any other condition that raises venous pressure may impair CSF absorption as well. Impaired absorption is the cause of communicating hydrocephalus, in which flow from the lateral ventricles to the foramina of Luschka and Magendie is not obstructed. Blockage of CSF pathways is the most common cause of hydrocephalus in children and is often located at the narrow aqueduct of Sylvius proximal to the fourth ventricle and is referred to commonly as aqueductal stenosis. Conditions that can cause obstruction are intraventricular bleeding or scarring, tumors, or congenital malformations. Dandy–Walker cysts cause obstruction of the foramina of Luschka and Magendie and therefore may result in enlargement of all four ventricles.
Equipment
Different types of CSF shunts, which vary mostly by the location of the distal tubing and the type of reservoir or valve system, are available. The choice of CSF shunt type and the method of placement (endoscopically or nonendoscopically) depend on the individual patient’s anatomy and cause of hydrocephalus and the experiences and preferences of the neurosurgeon performing the procedure. Commonly, the patient or caregiver knows the location and type of shunt and is able to provide details regarding prior shunt placement and problems. Palpation of the hardware and plain radiographs may be used to acquire more information regarding the specific location of the shunt components. Most CSF shunts have the following components: (1) proximal shunt tubing, (2) reservoir system, and (3) distal shunt tubing (Fig. 143.3). Occasionally, the system will not contain a reservoir and instead will have a one-way valve.
The proximal shunt tubing has a fenestrated tip that is usually located in the ventricle but may also be located inside a communicating cyst or in the lumbar subarachnoid space. This tip allows free passage of CSF into the shunt system. More than one proximal catheter may be present if multiple, noncommunicating areas of the brain require shunting. The reservoir system consists of one or two “domes” or “bubbles.” Reservoirs may be placed directly over or slightly distal to the burr hole. This information is crucial when emergent access to the burr hole is needed. The distal shunt tubing leads from the reservoir unit to a part of the body that can accept the drained CSF, usually the peritoneum. The distal tubing may also be located in the vascular system or pleural cavity. Ventricular–atrial shunts are less commonly inserted because of the serious infectious complications that have occurred with these types of shunts but may be necessary due to severe scarring in the peritoneum. All modern shunt tubing is made of 1⁄8-in diameter Silastic elastomer, which causes minimal omental reaction and is resistant to cracking.
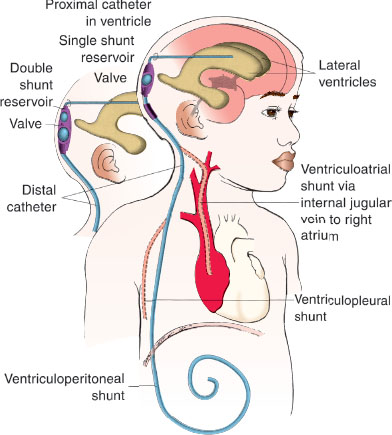
FIGURE 143.3 Diagram of typical ventriculoperitoneal shunt.
CSF shunt systems contain a one-way valve to prevent backflow of CSF into the ventricles. These valves are designed to operate at high, medium, or low pressure. Externally programmable valves, which can vary the opening pressure setting, are also available. An antisiphon device may be inserted into the distal portion of the system to prevent overdrainage of CSF and concomitant low-pressure complications.
Clinical Findings/Management
Mechanical Malfunction
Malfunction of a CSF shunt can be caused by the obstruction of the catheter lumen or disconnection of the various components. The proximal catheter lumen is usually obstructed by choroid plexus, but floating debris or hypercellular CSF can result in the same obstruction; the distal catheter can be obstructed by the surrounding omentum or can be kinked or coiled awkwardly impeding smooth drainage. Both proximal and distal portions can be occluded by the products of infection or by migration of the catheter tip into the brain parenchyma or intra-abdominal structures. Particularly in neonates, poor absorption of excess fluid in the peritoneum due to decreased surface area can create the appearance of luminal obstruction. In addition, as the child grows, the tension on the shunt system can lead to disconnection of the distal tubing.
Up to 60% of patients with CSF shunts experience a shunt malfunction in their lifetime, most commonly within the first 6 months of initial shunt placement. Parental history is paramount in deciding whether a child is experiencing symptoms of shunt malfunction. The parent often notices that the child “just isn’t acting right” or is less active or thinking less clearly than usual. The statement, “This is exactly how he acted the last time his shunt was obstructed,” is suggestive of another malfunction, regardless of the presence or absence of the symptoms listed in the following section.
TABLE 143.3
CONCERNING FINDINGS IN PATIENTS IN CEREBROSPINAL FLUID SHUNT MALFUNCTION

Common signs and symptoms of mechanical shunt failure include headache, visual disturbances, vomiting, lethargy, and irritability (Table 143.3). The astute parent or clinician may note mild ataxia, increased head circumference or bulging fontanel in an infant, swelling at the reservoir site, poor cognition, or abnormal behaviors. A classic sign is “sunsetting eyes,” which is really an upgaze paresis and eyelid retraction associated with Parinaud syndrome from pressure on the quadrigeminal plate by a dilated suprapineal recess in direct communication with the third ventricle. Increased tone, hyperreflexia, or Babinski reflex represents stretching and disruption of the corticospinal fibers originating from the motor cortex and can suggest shunt malfunction in a patient with a previously normal examination, although these symptoms are rarely present in a child without a severe alteration of consciousness and thus add little to the diagnosis. Patients with true, raised intracranial pressure (ICP), as manifest through Cushing’s triad (hypertension, bradycardia, and abnormal respiratory pattern), require immediate maneuvers to decrease ICP and guide them quickly toward operative repair of the shunt. Seizures are uncommon as the sole manifestation of CSF shunt malfunction. However, seizures can occur in children who have predisposing brain lesions, and many patients with CSF shunts have epilepsy. Shunt infection must be considered in the child with symptoms of shunt malfunction, especially if the child has a history of recent shunt revision. Ronan et al. reported that more than one-third of patients with shunt infection presented with symptoms of malfunction.
If the history and physical examination of the ill child with a CSF shunt suggests a possible shunt malfunction, further evaluation includes urgent neuroimaging with either noncontrast computed tomographic (CT) scan or MRI, with comparison to the most recent prior study, if available. MRI will likely reset a programmable shunt, so one should have access to a programming magnet if MRI is used in a patient with programmable shunt. A plain radiograph of the skull, chest, and abdomen (“shunt series”) is helpful both in assessing the integrity of the shunt connection and in identifying the components of the working system. The clinical suspicion of a shunt malfunction based on history and physical examination may outweigh the data obtained from radiographic studies because shunt failure may occur without radiographic signs.
Much discussion and controversy surround the clinician’s ability to assess CSF shunt function by “pumping” the shunt reservoirs. In a single reservoir system, this procedure involves depressing the reservoir bubble. Resistance to depression suggests distal catheter malfunction. Poor filling suggests either proximal catheter malfunction or small ventricles. The maneuver in a double-bubble shunt requires the initial depression of the proximal bubble, depression of the distal bubble to check for resistance, and subsequent release of the proximal bubble to check for poor filling. Pumping the shunt to test for obstruction is not always reliable. Piatt found that this maneuver had a positive predictive value of 21% and a negative predictive value of 78% in patients for whom the diagnosis of shunt patency or malfunction was definite. In addition, pumping of the shunt can cause entrapment of choroid plexus in the proximal shunt tubing and lead to proximal catheter obstruction where none previously existed.
If subsequent evaluation is still necessary to diagnose malfunction, a neurosurgeon should be consulted. It may be necessary to “tap” the shunt (Fig. 143.4). The patient’s hair is either shaved or trimmed. The scalp is cleansed first with alcohol and then with three applications of Betadine that are allowed to dry after each application. The shunt tap is performed by inserting a 23- or 25-gauge butterfly obliquely into the reservoir and holding the butterfly tubing perpendicular to the floor. The height of the CSF rise into the butterfly tubing, measured in centimeters, is the ICP. Normal pressure is between 5 and 10 cm; pressure of more than 20 cm is indicative of distal shunt malfunction requiring urgent revision. Slow or absent flow from the proximal reservoir (especially with occlusion of the distal reservoir of a double-reservoir shunt) is highly predictive of proximal shunt obstruction. In this case, the physician may notice that the reservoir collapses when gentle suction is applied to the butterfly with a syringe. It is important to avoid further suctioning of this reservoir because this could lead to aspiration of debris into the proximal catheter, causing a blockage where one did not previously exist. Poor flow during the shunt tap can also indicate slit ventricles and is therefore rarely the only data required to commit a patient to an operative shunt revision.
The shunt tap can be therapeutic and diagnostic. The child with a distal shunt obstruction or partial proximal obstruction may be eligible for urgent, rather than emergent, shunt revision if symptoms of increased ICP are alleviated after the tap. However, removal of too much fluid should be avoided because abrupt fluid shifts within the cranial vault can lead to disruption of subdural vessels. It is prudent to remove just enough fluid to decrease the ICP below 20 cm and repeat the procedure if symptoms return before definitive surgical management.
The child with complete obstruction of the proximal catheter does not obtain relief of symptoms after a shunt tap because the obstruction prevents adequate aspiration of fluid from the ventricles. These children usually respond temporarily to medical management that decreases their ICP; however, it should be stressed that restoration of shunt integrity and function is the permanent treatment of shunt obstruction. This treatment includes the administration of acetazolamide (Diamox) 30 to 80 mg/kg/day and Decadron 1 mg/kg/day and hyperventilation in the unstable patient. If the child is experiencing life-threatening symptoms from proximal obstruction, is unable to undergo immediate surgical repair, and is unresponsive to medical management, a burr-hole puncture procedure may be performed (Fig. 143.5) only in dire circumstances, as the procedure carries with it life-threatening risks such as disruption of intraparenchymal vessels and tissue. By nature of the procedure itself, the proximal shunt catheter is torn and urgent revision is therefore mandatory. The burr hole is best identified by direct palpation and confirmation with the skull radiographs. For example, a Rickham reservoir is located directly over the burr hole, whereas standard shunt valve/reservoir systems are usually, although not always. A 3½-in spinal needle is inserted perpendicular to the skull through the burr hole to a depth of no more than 5 cm. After the stylet is removed, fluid should drain spontaneously and should be allowed to do so until flow slows down. The patient’s condition should stabilize sufficiently for transport to an operating suite or tertiary care institution.

FIGURE 143.4 Tapping the cerebrospinal fluid shunt.
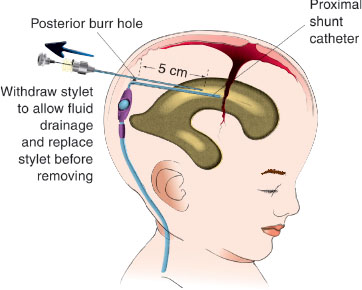
FIGURE 143.5 Burr-hole puncture.
Another method of temporarily relieving a lumen obstruction is to flush a small amount of sterile saline through the clogged tubing in an attempt to dislodge the obstruction. This method can be used for distal or proximal obstructions, with the caveat that instilling a few more milliliters into the ventricles may in fact worsen the patient’s condition. In this procedure, the double-bubble reservoir that is not being used must be compressed to allow the fluid to go in only one direction.
In an infant with an open fontanel, the physician can aspirate fluid through a direct ventricular puncture (Fig. 143.6)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







