
Tachydysrhythmias
Scott R. Votey and Eric Silman
The axiom that we must treat patients and not electrocardiograms (ECGs) or laboratory results is perhaps nowhere more relevant than in the emergency management of tachydysrhythmias. Decisions must be guided principally by the clinical presentation, and exact rhythm identification should be subservient to the goal of prompt appropriate management.
The acute and chronic management of tachydysrhythmias differ importantly. Acute management focuses on stabilization and typically involves emergent administration of intravenous (IV) medications or external electrical cardioversion, while chronic management increasingly relies on more advanced interventions which are beyond the scope of emergency medicine. This chapter focuses almost exclusively on acute management.
TACHYDYSRYTHMIA PATHOPHYSIOLOGY
Under normal circumstances, the sinoatrial (SA) node is the pacemaker for the heart. Depolarization is initiated in the SA node, and the impulse is conducted through the atrium to the atrioventricular (AV) node. Atrial depolarization corresponds to the P wave on the ECG. Conduction to the ventricles is slowed in the AV node, which provides a measure of protection against hemodynamically unstable tachycardias. The normal slowing in the AV node is seen on the ECG as the PR interval. After the impulse traverses the AV node, it is conducted first down the His bundle, followed by the left and right bundle branches, and then dispersed to the ventricular myocardium via the Purkinje fibers. Ventricular depolarization corresponds to the QRS wave on the ECG. Normal impulse conduction produces a narrow QRS complex.
Tachycardias can be generated from any focus in the heart, because essentially all myocardial tissue has intrinsic pacemaker activity. There are two principal mechanisms by which tachydysrhythmias arise: reentry and enhanced automaticity. Reentry is a phenomenon in which an initial depolarizing impulse travels a closed-loop path that returns cyclically to its point of origin and, in doing so, generates a regular sustained tachydysrhythmia. The requirements for a reentrant tachydysrhythmia are (a) a closed-loop conduction path; (b) slower conduction and prolonged refractoriness in one limb of the loop; (c) propagation of the initiating impulse down the faster limb of the loop, with antegrade conduction down the slower limb blocked; and (d) retrograde excitation of the initially blocked slower limb, returning the impulse to its point of origin, where depolarization is reinitiated. Repetition of this cycle sustains the dysrhythmia with a premature beat as the typical precipitant.
Enhanced automaticity is an increase in the rate of the spontaneous depolarization of myocardial cells above their electrical threshold. Sinus tachycardia is the physiologic response to enhanced automaticity of the SA node. When enhanced automaticity results in more rapid depolarization of extranodal myocardial cells, these foci can produce ectopic tachydysrhythmias. Examples include paroxysmal atrial tachycardia (PAT) and junctional tachycardia. Enhanced automaticity often occurs as a result of increased sympathetic tone (e.g., with fever, thyrotoxicosis, or sympathomimetic overdose). Treatment of these dysrhythmias generally should be directed at removing the underlying stressor.
CLINICAL PRESENTATION
Patients with tachydysrhythmias can present with wide-ranging signs and symptoms, from palpitations to cardiac arrest. Most, however, complain of some constellation of palpitations and dizziness. Syncope is an important presenting complaint of patients with any arrhythmia and should prompt cardiac rhythm evaluation in the emergency department (ED). Up to 70% of patients with tachydysrhythmias complain of chest pain, but it important to remember that not all chest pain in this situation reflects myocardial ischemia. Chest pain in a young otherwise healthy patient with a supraventricular tachycardia (SVT) rarely signifies ischemia, whereas the chest pain of an older patient with reasonable likelihood of coronary artery disease should be taken seriously. Other symptoms due to the dysrhythmia such as nausea and diaphoresis also overlap with those of myocardial ischemia. Symptoms of tachydysrhythmias depend on the hemodynamic consequences of the rate and rhythm, with those patients who are hypoperfused presenting with severe symptoms such as syncope, chest pain, or cardiac arrest. Hypotension and poor perfusion are more typically dependent on the heart rate produced by any dysrhythmia than they are on the source of dysrhythmia. Ventricular tachycardia (VT), for example, conjures up images of imminent catastrophe, and SVT is usually assumed to be benign. However, some patients with VT are relatively stable, at least in the short run; whereas, in other patients, SVT can be acutely life-threatening.
ED EVALUATION
In the ED, where diagnostic data are often limited and the patient’s condition warrants prompt action, tachydysrhythmias are most successfully managed using an algorithmic diagnostic and therapeutic schema that is easily remembered and applied. The following approach is broadly applicable to the emergency management of tachydysrhythmias.
Step 1. Be Prepared for a Cardiopulmonary Arrest
Any patient with a tachydysrhythmia is at risk for deterioration, because of either the dysrhythmia or its treatment. Being prepared for a cardiopulmonary resuscitation by having advanced airway equipment and a defibrillator at the bedside is essential.
Step 2. Determine Stability
Determine what hemodynamic effect the rhythm is having on the patient. In this context, unstable is defined as a heart rate and blood pressure inadequate to maintain vital organ perfusion and function, manifested clinically by significant chest pain, pulmonary edema, altered mental status, syncope, or severe hypotension. Categorizing a patient as stable or unstable is complicated by the innumerable gradations on the continuum from asymptomatic health to cardiopulmonary arrest. Altered mental status and ischemic chest pain with ECG changes of ischemia are convincing evidence of instability. Electrical cardioversion should be used to treat unstable patients with any tachydysrhythmia except sinus tachycardia and multifocal atrial tachycardia (MFAT).
Blood pressure is one of the most commonly used determinants of patient stability. It is important to remember that arbitrary blood pressure cutoffs for defining stable or unstable do not take into account the patient’s baseline blood pressure. A systolic blood pressure of 90 mm Hg is of far more concern in a hypertensive elderly patient than in a young and healthy 20-year old. It should be emphasized that stability is subject to change on a minute-to-minute basis, and therapy must be adjusted accordingly.
After clinical stability is assessed, the ECG should be rapidly and systematically evaluated. Although most therapeutic decisions can be made on the basis of a rhythm strip, it is prudent to obtain a 12-lead ECG as soon as it is feasible. A 12-lead ECG can help determine if myocardial ischemia is present and can aid in differentiating among tachydysrhythmias, especially between sinus tachycardia and other regular SVTs.
Step 3. Determine the Rate
The more extreme the ventricular rate, the more likely the patient is to become unstable. Sinus tachycardia rarely exceeds 160 to 180 beats/min in adults. Although SVTs typically have rates slower than 180 beats/min, they can be faster in the presence of a bypass tract. VT is not slowed by passage through the AV node and can thus be much faster (250 to 300 beats/min) but is usually less than 220 beats/min.
Step 4. Determine the QRS Complex Width
When emergent cardioversion is not required, subsequent decisions can be based on the duration of the QRS complex. In an adult, a QRS width of greater than 0.12 ms is considered “wide.” In many adults with a wide-complex tachydysrhythmia, the QRS duration is substantially greater than 0.12 ms. In children, the QRS duration is age dependent, but any QRS duration >0.10 ms is considered prolonged. It is usually possible to determine if a rhythm is wide or narrow from a rhythm strip alone, but if there is uncertainty, the widest QRS complex in the 12-lead ECG should be used as the measure.
Narrow-complex tachycardias can be assumed to be supraventricular. Wide-complex tachycardias are the result of any of three distinct pathophysiologic processes:
1. The rhythm originates in the ventricle and does not use the normal conduction pathway.
The origin of the tachycardia is supraventricular, but there is a block of the normal conduction pathway below the AV node (bundle-branch block), often referred to as “aberrancy.”
2. The origin of the tachycardia is supraventricular, but there is an accessory conduction pathway that bypasses the normal conduction pathway.
With rare exceptions, a wide-complex tachycardia should be treated as VT for three reasons: (a) VT is the most common cause of a wide-complex tachycardia; (b) despite the existence of diagnostic criteria, VT cannot be reliably distinguished from SVT with aberrancy in the clinical setting (1), and treating VT as SVT can be fatal (although treating SVT as VT is not harmful); and (c) even if the tachycardia has a supraventricular origin, there may be an associated bypass tract, in which case, some standard SVT treatments (e.g., verapamil) are contraindicated. It is never safe to assume that a wide-complex tachycardia is caused by aberrancy.
Step 5. Assess the Regularity of the RR Intervals
An irregular narrow-complex tachycardia is usually caused by atrial fibrillation (AF), although it can occasionally be caused by MFAT or atrial flutter with variable AV block. Because VT is usually regular except during the first few beats, an irregular wide-complex tachycardia should also raise the suspicion of AF with a bundle-branch block (AF-BBB) or a bypass tract. Distinguishing which patients have AF with a bypass tract is important, because the treatment of choice for these patients is cardioversion, even if they are stable. Although this differentiation can be difficult, two characteristics are helpful. In contrast to patients with AF-BBB, patients with AF with a bypass tract may have (a) very rapid conduction, with some RR complexes intervals equivalent to >250 beats/min, and (b) bizarre morphology QRS complexes resulting from fusion and partial fusion of beats from normal AV and bypass-tract conduction.
Step 6. Determine the Presence or Absence of P Waves
Determine whether P waves are present, their morphologic characteristics, whether they are regular, and how they are related to the QRS complexes. Regular uniform P waves preceding the QRS complex help distinguish sinus tachycardia from other SVTs. A normal P wave axis is one with an upright P wave in lead II and negative in aVR. This axis strongly suggests the atrial activity is coming from the SA node and is indeed sinus in nature. Although similar P waves may be present with the much rarer PAT, an abnormal P wave axis or morphology should alert one to the ectopic origin of that rhythm. An irregular narrow QRS-complex rhythm with more than three P wave morphologies is indicative of MFAT. One should, in addition, look for the regular sawtooth deflections of the ECG baseline that characterize atrial flutter waves.
Application of this scheme organizes dysrhythmias into four major categories based on the ECG appearance, with the additional distinction of clinically stable or unstable. The four categories are (a) narrow complex, regular, P waves present or absent; (b) narrow complex, irregular, P waves present or absent; (c) wide complex, regular, no P waves; and (d) wide complex, irregular, no P waves. Generally, each of the specific dysrhythmias that fall into each of these categories is treated in the same fashion, thus the simplicity of the approach. Occasionally, unstable patients need treatment initiated before analysis of the rhythm can be completed. In addition, once the rhythm is stabilized, the patient may require additional pharmacologic treatment to prevent recurrent dysrhythmias.
KEY TESTING
• Cardiac monitor strip
• 12-lead electrocardiogram
• Chest radiography*
• Chemistry*
• Cardiac enzymes*
*In select patients based on particular dysrhythmia and underlying comorbidities and risk factors.
DIFFERENTIAL DIAGNOSIS AND ED MANAGEMENT
Narrow-Complex Tachycardias
Narrow Complex, Regular, P Waves Present
Sinus tachycardia makes up the vast majority of these cases. Tables 85.1 and 85.2 outline the medical and electrical therapy for patients presenting with a wide variety of tachydysrhythmias. PAT is a rare alternative diagnosis. Therapy for sinus tachycardia is directed at the rhythm’s underlying cause rather than at the rhythm itself. For example, patients with sepsis and sinus tachycardia or those with tachycardia due to hemorrhage require volume resuscitation and those with alcohol withdrawal or sympathomimetic intoxication benefit from benzodiazepines. There are a few conditions in which pharmacologic slowing of sinus tachycardia are beneficial. For example, β-blockers are important in thyroid storm to counteract the adverse consequences of extraordinary increases in sympathetic drive. While it was once standard to administer β-blockers to tachycardic patients with myocardial ischemia to decrease myocardial demand, routine early IV β-blockers are no longer recommended in select ACS patients because of a lack of clear mortality benefit and an increased risk of cardiogenic shock in some patient groups (2).
TABLE 85.1
Selected Drugs for Tachydysrhythmias
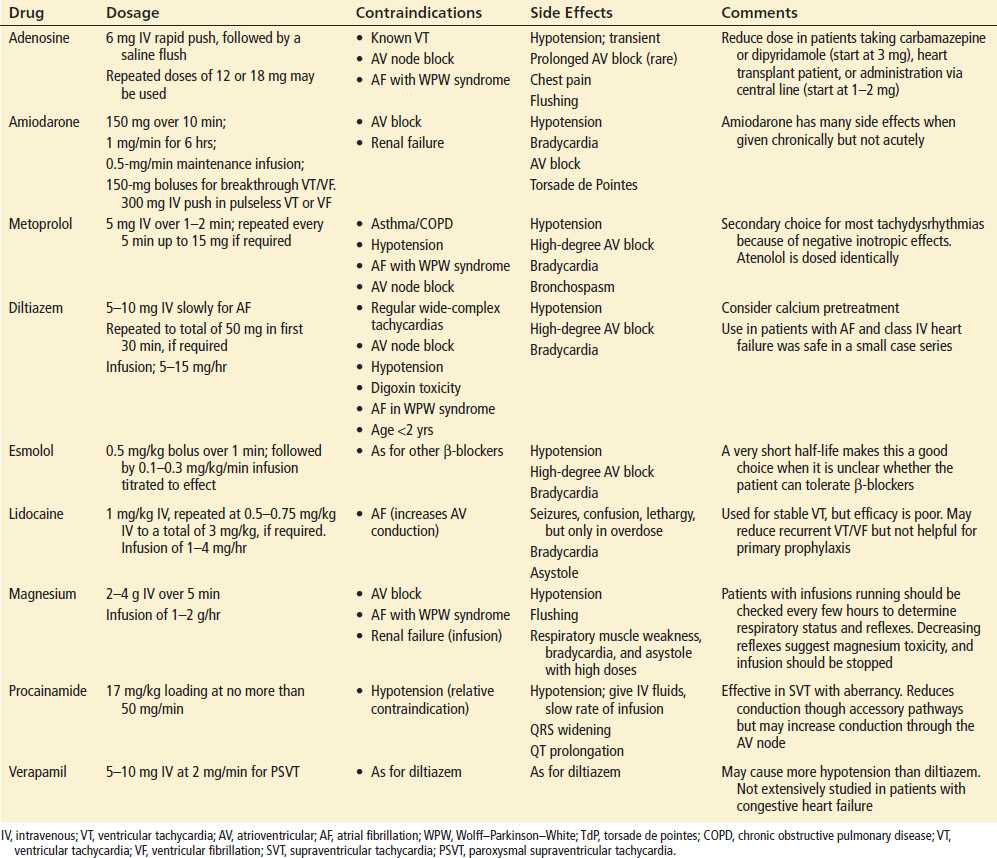
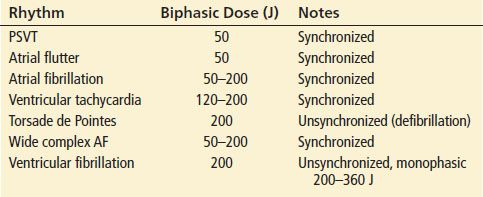
TABLE 85.2
Electrical Therapy of Tachydysrhythmias
Narrow Complex, Regular, No P Waves
The differential diagnosis includes paroxysmal supraventricular tachycardia (PSVT) and atrial flutter with a consistent AV block. Generally all these reentrant rhythms are treated in the same way with blockade of the AV node.
Paroxysmal Supraventricular Tachycardia
PSVT (Fig. 85.1) makes up the majority of cases in this category. The two main types of PSVT are atrioventricular nodal reentrant tachycardia (AVNRT) and atrioventricular reentrant tachycardia (AVRT), the differentiation of which is not important in the ED.
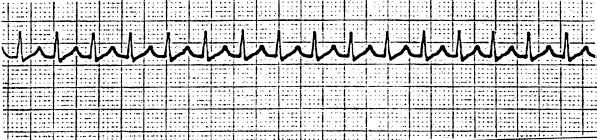
FIGURE 85.1 Paroxysmal supraventricular tachycardia.
Patients with PSVT who have severe signs of end-organ hypoperfusion should be cardioverted; more than 90% convert with small doses of electrical energy (≤50 J). Pharmacologic treatment of PSVT, typically a reentrant tachycardia in which the AV node is part of the circuit, is directed at blockade of the AV node. Vagal maneuvers may be tried first: carotid massage, the Valsalva maneuver, and ice-water immersion work well in many patients. However, because many patients with recurrent PSVT are familiar with vagal maneuvers, they often present for treatment only when these maneuvers have failed; therefore, these maneuvers are only occasionally effective in the ED.
Treatment options include adenosine, verapamil, diltiazem, and β-blockers. Adenosine, because of its brief duration of action and its resultant lack of sustained hemodynamic effect, is the drug of choice. The recommended initial dosage of adenosine is a 6-mg bolus over 1 to 3 seconds, followed by a rapid saline flush. If there is no response in 1 to 2 minutes, a 12-mg dose can be administered in the same manner. The use of doses of adenosine upward of 24 mg as a bolus have been reported in the literature, but are only marginally more effective than 12 mg. It is important that the drug be given as a rapid bolus to achieve high levels in the heart. In fact, adenosine is degraded so rapidly in plasma that the most proximal IV access possible (usually an antecubital vein) is desired.
In the absence of any of the standard contraindications to calcium channel blockers (hypotension, congestive heart failure, SA or AV nodal disease, or the previous use of IV β-blockers), diltiazem is a safe and effective alternative to adenosine. The standard initial dose is 10 mg IV and most patients convert to sinus rhythm within about 2 minutes. Incremental doses up to a total of 50 mg in the first 30 minutes are usually successful in those who do not. Verapamil is also effective. There is a substantial incidence of adverse effects when contraindications are present, so they should not be ignored. Many pediatric cardiologists consider it unsafe to use verapamil in children younger than 1 year of age and use adenosine exclusively in this age group.
Esmolol, an extremely short-acting β-blocking agent with a half-life of <10 minutes, is moderately effective in treating PSVT, although success rates are significantly lower than with either adenosine or verapamil and the incidence of hypotension is higher. Esmolol is, therefore, a secondary drug in the treatment of PSVT.
Atrial Flutter
Atrial flutter is a macroreentrant arrhythmia in which the circular current flow produces characteristic “sawtooth” waves, best seen in leads V1 and II, III, and aVF (Fig. 85.2). Atrial rates between 260 and 300 beats/min are typical, and the most common AV block of 2:1 produces ventricular rates of approximately 150 beats/min. Variable AV block can cause an irregular narrow complex rhythm. The ED management of atrial flutter is similar to that of AF. The decision to perform electrical cardioversion or attempt chemical rhythm control versus rate control must be made in the context of many factors: clinical stability, patient comorbidities and associate stroke risk, as atrial flutter carries a similar risk of stroke and many patients in atrial flutter also suffer from AF.
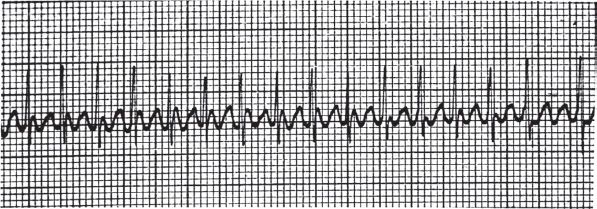
FIGURE 85.2 Atrial flutter.









