Fig. 12.1
Rationale and sequence of chapter. The headings, subheadings, and sections are displayed in columns from left to right
- I.
Heart Transplantation
- A.
Isolated heart donor harvest for heart transplantation.
- B.
Combined Donor Harvest of the Heart and Lungs for Distinct Recipients
- C.
Heart Implantation Technique: Primary Operation
- D.
Heart Implantation Technique: Reoperative Surgery
- E.
Heart Implantation Technique: Bridged Patients
- A.
- II.
Heart–Lung Transplantation
- A.
Combined Heart and Lung Harvest for Heart–Lung Transplants
- B.
Combined Heart and Lung Implantation: Primary Operation
- C.
Combined Heart and Lung Implantation: Reoperative Surgery or Surgery in a Potentially Hostile Pleural Space
- A.
For each of these procedures, safeguards and pitfalls are enumerated. Anesthesia management, preoperative care, and postoperative care are discussed in other chapters in this textbook, and so will not be addressed here in any depth. The reader is referred to these chapters for further information. The reader is also referred to Donald McRae’s riveting account of the race to perform the world’s first heart transplantation for further historical context [3].
Heart Transplantation
The donor operations are herein described for an isolated heart harvest and a combined heart and lung harvest for separate recipients; a description of the en-bloc harvest of the heart and lungs for a single recipient will be discussed in Sect. “Combined Heart and Lung Harvest for Heart–Lung Transplants”. The cardiac recipient operation is discussed under this heading for three separate scenarios: primary implantation, reoperative implantation, and implantation in patients bridged with a left ventricular assist device (LVAD) .
Historically, the cardiac implantation techniques have been classified by the type of atrial connections constructed: classical biatrial implantation, bicaval implantation, and total heart implantation (see Fig. 12.2). The last procedure [4], where almost all the recipient atrial tissue is excised, is infrequently used because of the additional ischemic time required without a demonstrable benefit. Therefore, it has been largely abandoned and will not be discussed further here. The reader is referred elsewhere for the technical details of this operation [4].
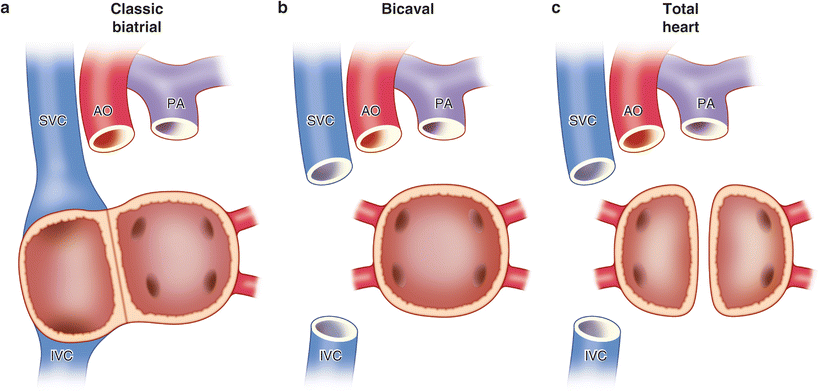

Fig. 12.2
The three different types of orthotopic heart implantation based on atrial connections. (a) Classical biatrial implantation. (b) Bicaval implantation. (c) Total heart implantation
The relative timing of the donor and recipient operations is planned so as to minimize ischemia time, defined as the interval from donor cross-clamp application to recipient cross-clamp removal. The best outcomes are achieved with ischemia times under 4–6 h, although longer times are acceptable depending on the clinical scenario. Figure 12.3 illustrates the complexity of coordinating the donor and recipient procedures. A summary of the sequential steps for the donor and recipient tracks, as well as the communication requirements, are depicted. A general rule of thumb we adopt is, “When in doubt, make the phone call.” I suppose in the modern era we can modify that rule of thumb to the following: “When in doubt, send the text.” Precise timing is paramount and miscommunications will be to the detriment of the recipient.
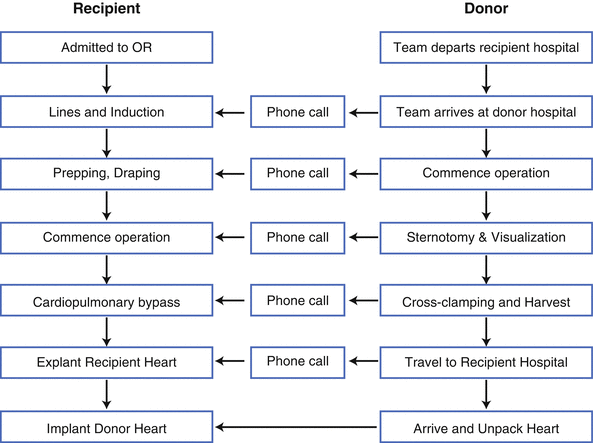

Fig. 12.3
Coordination of the recipient (left) and donor (right) operations. Vertical arrows indicate progression of the recipient and donor tracks, short horizontal arrows indicate communication opportunities between the tracks, and a longer horizontal arrow indicates the fusion of the donor and recipient tracks
Ideally, the donor heart should arrive in the recipient operating room just as the recipient team is ready to go on cardiopulmonary bypass (CPB) . It is therefore critical to plan the timing as meticulously as possible. This is usually undertaken by working back in time from the planned timing of the actual heart implantation to the initial events on the donor and recipient tracks so as to create a timetable template, as illustrated in Fig. 12.3. The most important recipient characteristics that play a role in the timing include whether the recipient is hospitalized or at home, requires a cross-match, is on oral anticoagulation therapy, has had prior cardiac surgery, and/or is on mechanical circulatory support (MCS) . The traditional cross-match is usually the component that requires the most time, since tissue must travel from the donor hospital to the recipient hospital prior to a final decision regarding whether to accept the organ. Some centers have started adopting virtual cross-matching of HLA antibodies, but this is not universal at present for thoracic organ transplantation [5, 6]. The most important donor characteristics that play a role in the timing include the travel time between the donor and recipient hospitals and whether other donor organs are being procured. The actual scheduling of the donor operation is usually at the discretion of the donor hospital, so this will often dictate the actual timetable of the donor and recipient tracks. Unfortunately, because of the regularly scheduled operations at often busy donor hospitals, the donor harvest may be relegated to the nighttime hours.
The decision regarding whether to accept a specific remote donor based on projected ischemia time is rarely made in isolation. Other important factors influencing the acceptable ischemic time for a particular donor include the donor cardiac function, the degree of donor inotropic support, the recipient’s hemodynamic stability, and the likelihood that the recipient will get another heart offer in a reasonable time period. For example, a longer donor ischemic time may be acceptable for the unstable recipient if the donor heart function is excellent and the donor is on minimal inotropic support. There are thus few hard-and-fast rules, and decisions needs to be individualized.
Isolated Donor Harvest for Heart Transplantation
The Operation
Prior to departing for the donor hospital, the donor team reviews all the relevant data. In addition to all the background data on the donor, it is critical to confirm on multiple occasions the ABO compatibility of the donor and recipient. At the very least, ABO compatibility needs to be confirmed and documented at the following mileposts:
Upon initial donor online screen
During the initial phone conversation between the donor surgeon and the organ procurement organization on-site coordinator
At the time of arrival at the donor operating room, and
At the time the donor heart arrives in the recipient operating room.
An incompatible match is disastrous for the recipient, and therefore the foregoing “belt-and-suspenders” approach is essential.
During the travel to the donor hospital, ongoing communication occurs between the donor and recipient teams, as outlined in Fig. 12.3. This communication continually and repeatedly occurs during the entire process to ensure that timing gets resynchronized in an iterative fashion, since seldom is the initial timetable accurate. The goal, again, is to have the donor heart arrive at the time the recipient team is ready to go on CPB. Occasionally, hemodynamic instability in the recipient mandates going on CPB while the donor team is en route, but this is fortunately a rarity.
Upon arrival at the donor hospital, the donor team again reviews the data, confirms ABO compatibility, and directly visualizes the most recent echocardiogram and coronary angiogram, if available. If the heart is deemed acceptable at this point by the donor team, confirmation is communicated to the recipient team. In addition, revised times are agreed upon by the donor/recipient teams (see Fig. 12.3).
Given that many donor cardiectomies are performed at hospitals unfamiliar to the operating surgeon, it is critical for the cardiac surgeon to communicate with the anesthesia team regarding relevant parameters and mileposts during the harvest—e.g., where to maintain the mean arterial pressure and central venous pressure, what kind of volume to administer, what inotropic or pressor agents to use if necessary, when the central venous line should be withdrawn, and when disconnection from the ventilator is appropriate.
Typically, the donor harvest proceeds in two stages. First, the initial sternotomy, evaluation, and preparatory dissection are performed. Second, once the other organ harvest teams are ready, the heart is arrested, explanted, and prepared for transport.
Commencing the first stage, a time-out is performed and the donor is prepped and draped. The back table that will be used for preparing the heart for transport is set up with basins, three sterile bags, and a suitable container (I use a wall-suction container available in most units). The individual donor teams make their respective incisions (usually a continuous sternotomy and laparotomy incision). Once the sternotomy is performed, a retractor is inserted. If available, a retractor with sternal spikes is used, since it is common for nonspiked retractors to slide down towards the abdomen during the harvest. The spikes press into the marrow and keep the retractor from sliding along the long axis of the sternum. Hemostasis of the bone marrow with bone wax is helpful. Bleeding should be under tight control, since the operation may take several hours until cross-clamping occurs. Substantial blood and volume loss can occur during this interval if one is not meticulous.
The pericardium is opened, and stay sutures are placed circumferentially. Observation and palpation of the heart is then undertaken. Observation focuses primarily on cardiac function and the presence of scars or contusions. Each of the cardiac chambers and great vessels is palpated for thrills. In the modern era, it is rare for surprises to occur that were not apparent on the preoperative echocardiogram. If, however, any abnormality is noted, a TEE should be considered intraoperatively and results communicated to the implanting team. Next, the coronaries are palpated to confirm the absence of significant plaque or calcifications, especially if the patient has not had a left heart catheterization prior to the operation. Once the heart has thus been visualized and palpated, the recipient team is again contacted to let them know if the heart is acceptable.
It is important to remark that there is a tradeoff in the amount of dissection performed at the initial stage of the donor harvest. Generally speaking, once the initial cardiac dissection is complete, it takes at least an hour and sometimes more before the other teams are ready for cross-clamp application. Therefore, one needs to be cautious about causing any hemodynamic instability—either from myocardial dysfunction or inadvertent injury—during the initial dissection phase that would prompt premature cross-clamping.
Accordingly, the initial dissection is limited to relatively safe maneuvers, and will depend on the experience of the operating surgeon. The aorta and pulmonary artery (PA) are separated and freed up from their pericardial attachments. The aorta should be freed up to the arch. The superior vena cava (SVC) and inferior vena cava (IVC) are dissected away from their respective pericardial attachments, and care should be taken not to injury the azygous vein as it enters the SVC posteriorly. Clearing cardiac structures from the pericardium and from each other while the heart is full will facilitate the explantation when the heart is empty, since it is harder to find dissection planes when the heart is flaccid. Two silk ties are placed around the SVC above the azygous vein entry site. An additional tie is placed around the azygous vein if this can be done with ease. Umbilical tapes are passed around the aorta and the IVC. At this juncture, the donor heart team needs to pause for the abdominal organ teams to complete their preparatory dissection to be ready for cross-clamping. An estimate of the time remaining from the abdominal team is obtained, and this information is relayed to the recipient team, with adjustments made to the timetable.
Prior to leaving the operating table at the end of the first stage, the surgeon ensures that he has selected his cross clamp and has communicated his suture and other disposable needs for the second stage to the scrub nurse or technician.
Figure 12.4 shows the sequence of events for the second stage of an isolated heart harvest.
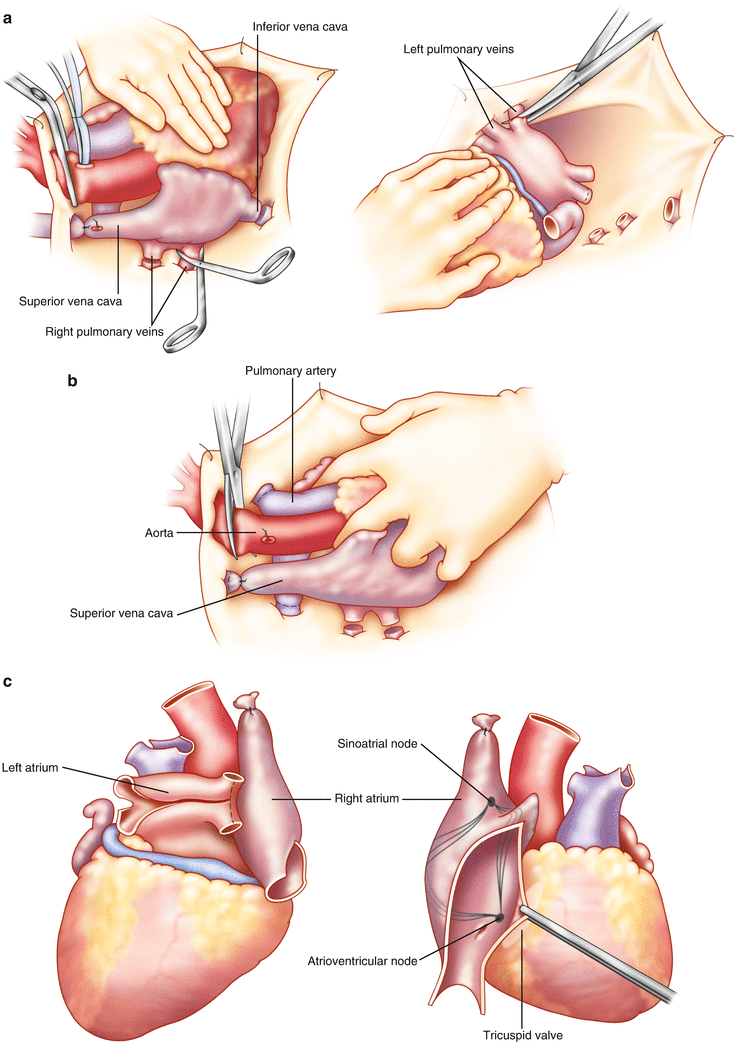

Fig. 12.4
Steps in the performance of the cardiac harvest . (a) The heart has been arrested and the cardioplegia administered. The SVC has been ligated, an incision made in the IVC, and the right sided pulmonary veins are divided at the pericardial reflection. With the heart retracted cephalad and to the right by the surgeon’s left hand, the left-sided pulmonary veins are divided. (b) With the surgeon’s left hand retracting the heart towards the patient’s feet, the branch pulmonary arteries are divided at the pericardial reflection and the aorta divided just proximal to the cross clamp. (c) At the back table, the back wall of the left atrium is opened, as is the pulmonary artery. In addition, for planned right atrial anastomosis, an incision is created from the orifice of the IVC towards the right atrial appendage, avoiding the sinus node
The second stage is launched once the abdominal team is near-ready for cross-clamping. A dose of 30,000 units of heparin is administered by the anesthesia team. Three minutes are allowed to pass to allow recirculation of the heparin. During this time, the cardioplegia bag (1–2 L) and line are prepared. The line is deaired and clamped at the field. A purse-string suture is placed in the mid-ascending aorta, and the cardioplegia cannula is inserted within the purse string. The purse string is snared down, the cannula is deaired and then attached to the previously deaired cardioplegia line. A pressure line may be attached if desired, but palpation of the aorta during cardioplegia administration usually suffices. The cardioplegia line should be kept clamped at the field to prevent inadvertent premature administration of cardioplegia prior to crossclamping.
An incision is made in the right edge of the pericardium at the level of the diaphragm down to abut the lateral aspect of the IVC. This incision will allow egress of blood return from the IVC and coronary sinus down into the right pleura, preventing rewarming of the heart. This maneuver is not performed if the right lung is being harvested.
Once the abdominal team is ready for cross-clamping, the cardiac surgeon asks the anesthesiologist to withdraw any central lines traversing the SVC. Two suction cannulae are prepared and placed in the mediastinum. These will be managed by the first assistant. The goal will be to clear the field of blood to (1) enable visualization of the heart and (2) prevent rewarming of the heart. Accordingly, one suction will be placed with its tip by the IVC, and the second, by the left inferior pulmonary vein (PV)—i.e., the sites where the initial venting incisions will be placed. The SVC is then ligated once, leaving the other ties to be secured after cardioplegia delivery. The location of the IVC hemisection site sometimes needs to be negotiated with the liver team; however, one must ensure that the coronary sinus is not injured by cutting too high. The coronary sinus can be protected by the surgeon’s placing his left hand with his index and middle fingers straddling the cavoatrial junction and then gently retracting cephalad. Once this hemisection is complete, a second hemisection is performed of the left inferior PV. Now, both the right and left ventricles are vented. Once the heart is decompressed after several beats, a cross clamp is applied to the ascending aorta distal to the cardioplegia line, and the cardioplegia is administered by the perfusion team. The aortic root should be palpated to confirm that the root is adequately distended. Again, a pressure transducer can be used if so desired. Further details regarding myocardial preservation are discussed in the next section (vide infra).
At cross-clamping, another phone call should be made to the implant center to resynchronize the time schedules (see Fig. 12.2).
As cardioplegia is being administered, ice slush is repeatedly placed on all surfaces of the heart, and blood and cardioplegia effluent is evacuated from the pericardial space. As stated above, one suction cannula is placed at the IVC incision, and the other at the left inferior PV incision. Besides making sure all the effluent is displaced from the heart, the surgeon must also pay close attention to the heart to ensure that it is not getting distended. The right heart may get distended if the IVC incision is too small; the left heart may get distended if aortic insufficiency is induced by the pressurized root or the left inferior PV incision is too small. Once the cardioplegia is completed, the cardioplegia purse-string and cannula are removed.
It is important to develop a standard sequence for excising the heart, since there are a variety of possible permutations. Since the heart is ischemic at this point it is imperative that the heart be kept as cool as possible and excised efficiently. My protocol is as follows: The assistant manages the suction cannulae, since blood will continue to pool in the field as the heart is being excised. The assistant is also responsible for adding slush to the heart intermittently to maintain preservation. It is generally a good rule of thumb to habitually excise as much of the aorta and SVC as possible, particularly if the recipient has congenital heart disease or the recipient operation is reoperative.
The first maneuver is to tie the remaining ties on the SVC and azygous vein. Once this is complete, the SVC is divided between its two ties, and the azygous vein is divided at its connection to the SVC. The tie on the SVC remnant in the patient is placed to minimize further blood return to the pericardial space. (It is helpful here if the SVC had already been dissected completely during the initial stage of the harvest.) Next, the surgeon places a slush-packed lap pad in his left hand, and gently lifts the acute margin of the heart towards the patient’s left shoulder. He will then retract the heart progressively cephalad as the IVC and then the PVs are transected (Fig. 12.4a). In isolated heart harvests—where the lungs are not being harvested—the PV transections should be placed at the pericardial edge, to maximize the length attached to the left atrium. Once all the PVs are divided, the heart is released. The surgeon places his left index and middle finger splayed around the aortic and pulmonic roots and gently retracts the heart towards the patient’s feet (Fig. 12.4b). The branch pulmonary arteries are now divided at the pericardial reflection. Next, the aorta is transected just distal to the innominate artery at the arch. Finally, the surgeon wraps his left hand around the base of the heart and great vessels and exerts slight upwards retraction towards the ceiling. The remaining attachments to the pericardium and mediastinum are now severed. Please see Fig. 12.4a–c.
For the reader’s convenience, Table 12.1 lists the basic differences in cannulation, preservation requirements, venting requirements, and incisions of the heart transplantation and heart–lung transplantation recipient operations.
Table 12.1
Heart vs. heart and lung procurement
Step | Isolated heart | Both heart and lung |
|---|---|---|
Cannulation | Aorta alone | Both aorta and PA |
Preservation | Heart alone | Both heart and lungs |
Venting | RA | RA and LA |
Left atrial incisions | PVs at pericardium | LA between sulcus and PVs |
PA incisions | Branch PAs at pericardium | Main PA at bifurcation |
Heart Preservation, Preparation, and Transportation
As stated previously, 1–2 L of cardioplegia is administered to the heart prior to excision. If any concerns are raised about the adequacy of the preservation—e.g., a moderately hypertrophied heart, delay in arresting the heart, or induced aortic insufficiency, then a second liter may be given at the discretion of the operating surgeon. There are several cardioplegia solutions available; I have the most familiarity with the University of Wisconsin (UW) solution (Belzer). Most centers, like our own, use an intracellular solution for the heart—i.e., one with a relatively high potassium concentration to induce a diastolic arrest of the heart. The components of UW solution are listed in Table 12.2.
Table 12.2
Contents of University of Wisconsin solution
Component | Concentration |
|---|---|
Sodium | 35 mM |
Potassium | 125 mM |
Magnesium | 5 mM |
Sulfate | 5 mM |
Phosphate | 25 mM |
Bicarbonate | 100 mM |
Raffinose | 30 mM |
Glutathione | 3 mM |
Adenosine | 5 mM |
Allopurinal | 1 mM |
Hydroxyethyl starch | 50 g/L |
Dexamethasone | 16 mg/L |
Insulin | 40 U/L |
Penicillin | 200,000 U/L |
The role of myocardial preservation solutions is to preserve the microvascular, cellular, and functional integrity of the heart. The main protective components of the cardioplegia solution are (1) hypothermia, (2) potassium to arrest the heart, (3) impermeants to prevention cellular swelling (e.g., lactobionate and raffinose), (4) magnesium to prevent calcium accumulation in the sarcoplasmic reticulum, and (5) free radical scavengers to prevent free radical injury. Experimental and clinical use of the UW solution, which is an intracellular (high potassium) solution that includes the above ingredients, have provided sufficient evidence to support the expectation of excellent myocardial preservation for at least 6 h of ischemic time [7].
Once the cardioplegia is completed and the heart excised, the heart is transposed to the back table that previously had been set up. The surgeon places the heart on a lap pad in a basin filled with iced saline, and the heart is then examined in the order of the normal pathway for blood flow: The RA, the tricuspid valve, the pulmonic valve, the LA, the mitral valve, and the aortic valve.
To facilitate the exam and the later implantation, the following steps are taken in the field. The atrial septum is investigated through the IVC. If a biatrial anastomosis is planned (vide infra), then the RA may be opened from the IVC orifice towards the RAA, veering anterior to the sulcus terminalis to avoid injury to the tail of the SA node. The TV can then be examined as well for its morphology. The coronary sinus is visualized as it enters into the RA to ensure there has been no injury to this structure during the IVC transection (see Fig. 12.4c).
The left atrium adjacent to the PA is separated from PA by severing the attachments connecting the two. The PA is then opened with an incision connecting the branch PAs, and the pulmonary valve is inspected. The LA incision is opened by bridging the orifices of the pulmonary veins. Further trimming of the aorta, LA, and PA is left for later at the time of the implant. The AV and MV are examined for their morphologies as well. As mentioned previously, there are not likely to be any surprises relating to the cardiac valves.
Upon examination of the interatrial septum, if a patent foramen ovale (PFO) is present, it is helpful to close this during the donor operation with 5.0 prolene; this should be tied on the RA side. All PFOs , no matter how small, need to be addressed, since the frequent occurrence of postoperative right ventricular dysfunction may lead to considerable hypoxemia from right-to-left shunting otherwise. The heart is then placed in a sterile bag with cold UW solution. The untied bag is then placed in a rigid container (e.g., a wall suction container) containing slush. The first bag is then de-aired and tied, and the container is filled to the rim with slush. The container is closed and then sequentially wrapped in two additional sterile bags contained ice. Each bag should be tied. The heart, along with its enveloping bags and container, are placed in an ice cooler and transported back to the implant center.
Communication between the teams continues at appropriate times during the transportation to continually resynchronize the timing (see Fig. 12.2).
In recent years, several centers have participated in trials of warm perfusion circuits to maintain blood flow to the coronary circulation after explantation and during the transportation process. The PROCEED II trial evaluated the TransMedics® Organ Care System Heart technology, and a marketing application has been submitted to the FDA. At this point it is too early to predict what role continuous warm perfusion will play in the future of donor heart preservation [8]. The possibility of resuscitating and reevaluating warm perfused hearts that had been considered marginal has generated enthusiasm among those in the field.
Combined Donor Harvest of the Heart and Lungs for Distinct Recipients
Modifications to the above steps are required if the lung is being harvested simultaneously for a different recipient(s). The focus in this section will be on how lung procurement for separate recipients impacts the cardiac procurement. For further details, please see Chap. 8. Prior to prepping and draping, a bronchoscopy is performed to evaluate the tracheobronchial tree and obtain a specimen for gram stain and culture. Upon entry into the chest, the pleura are opened first and the lungs examined. The heart is evaluated and prepared as previously described.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





