Fig. 33.1
Adolescent in the headrest (ProneView, Dupaco, Oceanside, CA). The potential pressure points include the forehead and chin. It is important to make sure that the weight of the head is distributed between these pressure points
Anesthesia was induced with propofol and fentanyl and tracheal intubation was facilitated with low-dose rocuronium (0.5 mg/kg). Intubation was atraumatic and two bite blocks (made of soft gauze) were inserted into the mouth between the molars to protect the tongue from impingement during the MEPs [11]. Care was taken to tape the oral endotracheal tube securely because the patient would be prone for 4–6 h for this procedure. Several large-bore intravenous lines and a radial artery catheter were inserted.
Anesthesia was maintained with the administration of 0.6 % isoflurane and 70 % nitrous oxide. Several intravenous boluses of fentanyl (50–100 μg) were administered throughout the maintenance phase of the anesthesia in order to provide a constant level of analgesia.
The neurophysiologist was asked to acquire MEPs by stimulating at the C1 and C2 scalp electrode positions rather than using the C3 and C4 positions. SSEPs and electromyography (EMG) monitoring was also planned for this case.
Discussion
Although most intraoperative neuromonitoring groups prefer a total intravenous anesthesia (TIVA ) technique and most centers if using volatile agents limit the total volatile plus nitrous oxide, there is evidence that using trains of 3–5 pulses (interstimulation interval, 2 ms) for MEPs will allow adequate neuromonitoring with a volatile gas technique as long as the total MAC is 1 or less for the majority of healthy patients with robust responses [12–14]. Other anesthetic drug combinations include a mixture of another volatile agent (desflurane or sevoflurane) with nitrous oxide up to 1 MAC (see Chap. 19, “General Anesthesia for Monitoring”) or a total intravenous anesthetic, which could consist of a narcotic, propofol, methohexital, ketamine, or dexmedetomidine with or without nitrous oxide. Our rationale for choosing for a volatile gas with nitrous oxide is that if neuromonitoring signals are lost or there is a surgical event that necessitated a “wake-up test,” rapid emergence from anesthesia can be easily achieved with the volatile anesthetics [15]. Monitoring the MEPs using the C1 and C2 scalp electrode position produces less activation of the masseter muscle than when the C3 and C4 electrode positions are used (Fig. 33.2).
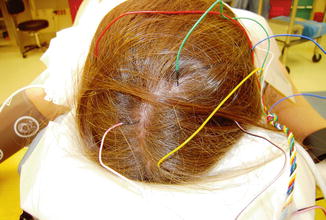

Fig. 33.2
View of neuromonitoring leads placed in a montage in the skin overlying the cortex
After induction of anesthesia, the patient’s blood pressure was 88/50 mmHg. The isoflurane concentration was decreased to 0.2 %. The blood pressure improved to 95/55 mmHg and the inhaled anesthetics were maintained at the lower concentrations. The patient was carefully positioned prone on the Jackson table and all extremities were padded and a forced hot air warmer blanket (Bair Hugger; Arizant Inc., Eden Prairie, MN) was placed on the lower extremities. The neuromonitoring technician reported good SSEPs and MEPs in all four extremities. The surgeon made the incision, the blood pressure rose to 130/88 mmHg, and the anesthesiologist increased the inhaled anesthetic concentration (1 MAC) and administered an additional bolus fentanyl for a total peri-induction dose of 15 μg/kg. The blood pressure decreased to a mean of 66 mmHg. The neuromonitoring technician then reported that there was a decrease in amplitude and increase in latency in the potentials from three extremities and loss of signal in the right hand for the SSEPs . The patient’s temperature at this time was recorded as 34.5 °C.
Discussion
The possible reasons for the decrements in the signal were identified and discussed. Although the initial plan of administering 1 MAC equivalent of isoflurane and nitrous oxide should have been compatible with neuromonitoring, it was determined that the baseline measurements were actually recorded when the patient was receiving 0.7 MAC. Volatile anesthetic drugs and nitrous oxide will decrease the amplitude and increase the latency of both the MEPs and the SSEPs. The MEPs are more sensitive to the effects of volatile agents. In neurologically intact adolescents, administering 1 MAC equivalents of isoflurane and nitrous oxide should be compatible with adequate signals. But in this case, 1 MAC of gas represented an increase in anesthesia from baseline and thus would be expected to cause the observed changes in latency and in amplitude. Since the attenuation of the signals occurred during the skin incision, it was deemed unlikely that the changes could be attributed to surgical injury to the spinal cord and nerves.
The anesthesia team decreased the inhaled isoflurane and nitrous oxide concentrations and started a propofol infusion of 75 μg/kg/min. This maneuver maintained the patient’s mean blood pressure between 65 and 70 mmHg. Since the modest increase in the inhaled isoflurane concentrations would not be sufficient to completely depress the SSEPs from the right hand, the technologist verified the placement of the electrodes and found them intact.
Within 5 min of decreasing the inhaled concentration of isoflurane and nitrous oxide , the SSEPs returned to their baseline values except for those on the right hand, which did not fully return and were only intermittent with prolonged latency and decreased amplitude. Coincidently, the pulse oximeter on the right index finger intermittently registered a weak signal.
Discussion
The different etiologies for the loss of SSEP signals from the right hand include loss of electrodes on the right hand, poor connection between electrode and skin of the right hand, injury to the spinal cord, and injury to the brachial plexus.
The technician checked the impedance of the electrodes on the patient’s right hand and found it to be normal at 5 kΩ. The recording and stimulating leads on the patient’s head, hand, arm, and shoulder were also intact. The arm position appeared to be normal. The anesthesia team informed the surgical team about the loss of signals and asked them whether there was any surgical manipulation of the lower cervical and upper thoracic spinal cord . The attending surgeon noted that surgical field included T4–L4 segments and did not include the high thoracic and low cervical area. The anesthesiologist peered under the drapes again and noticed that the right arm was abducted at the shoulder. At this point the orthopedic assistant surgeon admitted to “hip checking that arm out of the way” so they could better assist with the surgery. The anesthesiologist repositioned the arm so that the shoulder was not abducted but in a neutral position and that the upper arms were positioned 90° in front of the patient’s torso to limit traction on the brachial plexus. The anesthesiologist also established that the axilla was free from chest pads on the surgical table. The waveform on the pulse oximeter immediately normalized. Within 10 min, the SSEPs in the right hand returned to normal [16]. In this particular instance, both the pulse oximeter and the SSEP monitors revealed a positioning problem and possibly averted a postoperative neuropathy.
Decortication of the bone for the spinal fusion resulted in profuse venous bleeding and the surgeon requested that the anesthesiologist lower the mean blood pressure to 55 mmHg in order to stem the bleeding [17]. Although the anesthesia team had some concerns that the lower blood pressure might increase the risk of spinal cord and optic nerve ischemia, they concurred with the surgeon and lowered the blood pressure to a mean of 55 mmHg with labetolol [18]. Hypovolemia was rapidly treated by a 3:1 ratio of normal saline to blood loss fluid bolus. The patient’s temperature at this time was 34.5 °C and the hematocrit was 26 % with normal arterial blood gas values. The anesthesiologist turned on the fluid warmer and applied a forced warm air blanket to the upper torso to help maintain the patient’s temperature. Immediately, the neuromonitoring technician announced a deterioration in the monitoring signals. The anesthesiologist asked the neuromonitoring technician to characterize the recent change in signals and found that the amplitude and latency had not changed but that the signals became unreliable due to increased background electrical noise.
Discussion
Rapid fluid resuscitation with crystalloids can lead to excessive facial swelling, may interfere with organ function and can cause the patient to rapidly lose body heat in patients in the prone position. Both low body temperature and anemia are associated with increased latency and decreased amplitude in MEPs and SSEPs. However, electrical interference can lead to unreliable signals.
Electrical interference with the MEPs and SSEPs by other electrical devices commonly occurs in the operating room (Fig. 33.3). It is important to try to establish a temporal association between the addition of an electrical device such as a forced hot air warmer or a blood warmer with the onset of the electrical interference. The most efficient way to determine whether a device is causing interference is to briefly turn the device off and see if the interference resolves. Once the likely culprit is identified, often moving the device to a location in the room away from the neuromonitoring wires will decrease the level of electrical interference (see Chap. 16, “IOM Instrumentation Layout and Electrical Interference”).
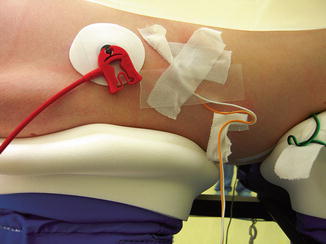

Fig. 33.3
Close proximity of the ECG lead and transversus abdominis neuromonitoring lead
The anesthesiologist turned off the blood warmer and forced air warmer and moved them to the contralateral side of the neuromonitoring wires. Although the electrical interference did not completely resolve, the signals were significantly improved [18, 19].
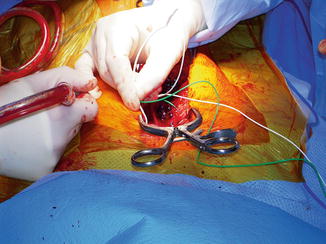
The surgeons inserted all their pedicle screws bilaterally. They then requested that the neuromonitoring technician help them do triggered EMG to ensure proper placement of the pedicle screws . The surgeons placed the stimulation probe on the pedicle screw and a needle electrode wire was inserted in the tissue above the surgical field to complete the circuit for the stimulation current (Fig. 33.4). The surgeon began to stimulate the first pedicle screw with 8 mA and gradually increased the current to 20 mA. No EMG signal was detected with stimulation.