Fig. 28.1
Anatomy of the posterior craniocervical junction in Chiari malformation (a). Sagittal noncontrast T1-weighted MR image in a patient with Chiari I malformation demonstrating herniation of the cerebellar tonsils (large arrow) below the foramen magnum. Also shown is the lamina of the second cervical vertebra (small arrowhead), the obex (small arrow), and the fourth ventricle (large arrowhead). Intraoperative posterior view of Chiari malformation surgery (top of photograph is caudal) (b). After a suboccipital craniectomy is performed and the posterior arch of C1 is removed, the posterior dural band (small arrowhead) is divided, revealing the descended cerebellar tonsil (large arrow) over the cervicomedullary junction (large arrowhead). The tonsillomedullary segment of the left posterior inferior cerebellar artery (small arrow) is shown coursing between the cerebellar tonsil and cervicomedullary junction
Positioning Considerations During Surgery
For the prone position, the head is held in three-point fixation. The neck is flexed to increase the working area in the craniocervical junction. It is imperative to recheck endotrachial tube positioning and ensure that bilateral breath sounds are present following neck flexion, as migration of the endotracheal tube leading to mainstem intubation is possible. An appropriate amount of neck flexion allows for surgical exposure without compromising adequacy of ventilation, arterial inflow, or venous outflow. The definition of excessive neck flexion may vary from patient to patient based on variability in boney anatomy or degree of preexisting neural structural compression. If arterial inflow is restricted, ischemia can result. If venous outflow is restricted, macroglossia and intracranial hypertension may occur. In general, there should be at least two to three fingerbreadths of space between the anterior mandible and the sternal notch. The shoulders are often gently pulled inferiorly and taped into position. Careful attention to the amount of traction on the shoulders can minimize the chance of brachial plexus injury. If somatosensory-evoked potentials are utilized for the case, the brachial plexus can be monitored.
Principles of Intraoperative Neuromonitoring for Chiari I Malformation Surgery
The body of evidence supporting mandated use of neuromonitoring during surgical decompression of Chiari I malformation is lacking. Clinicians have, nevertheless, demonstrated some utility in somatosensory-evoked potential (SSEP) and brainstem auditory-evoked potential (BAEP) monitoring during positioning and in assessing adequacy of surgical decompression respectively [17]. After establishing baseline SSEP signal values, changes during SSEP monitoring can indicate a problem with positioning of the patient due to compression of the brain stem or cervical spine. Prompt recognition and correction of the problem by repositioning can avert a potentially disastrous outcome. SSEP monitoring can also provide information about the dorsal sensory elements of the spinal cord and brainstem during the procedure. Likewise, direct mechanical surgical insults and ischemia of the brainstem due to compression, spasm, or injury to the PICA can be detected by changes during SSEP monitoring. Correction of the surgical insult, blood pressure manipulation, addition of corticosteroids, administration of rheologic agents, or repair of the injury may provide benefit if actively pursued in response to monitoring changes. Monitoring of MEPs is infrequently used during CM-I decompression as no clear advantage has been demonstrated beyond that offered by SSEP or BAEP monitoring. The use of MEPs in this setting should therefore be relegated to surgeon preference.
Case Illustration
Stroke During an Operation for Chiari I Malformation
A 28-year-old female presented with progressive debilitating Valsalva-induced suboccipital headaches and unilateral upper extremity weakness. She underwent MR imaging and was subsequently diagnosed with Chiari I malformation with cervical syringomyelia. The tonsils were noted to descend 7 mm below the foramen magnum, and a syrinx extending from C2 to C7 was appreciated. She elected to undergo suboccipital craniectomy, cervical laminectomy, cerebellar tonsillar reduction, and allograft duraplasty. The patient was positioned prone on laminectomy rolls, with the head and neck fixed in a three-point pin-based head fixation device. Two hours after skin incision, at the time of the cerebellar tonsillar resection, the posterior spinal radicular arteries on the dorsal aspect of the upper cervical spinal cord appeared blanched. This finding was believed to be unrelated to cauterization. Also within this time period, the neuromonitoring technician reported that the SSEPs were decreased in amplitude. With blood pressure elevation and the direct application of papaverine, vasodilation and reperfusion of the posterior spinal radicular arteries were noted within minutes of the above vascular changes. However, following reperfusion of the posterior spinal radicular arteries, the SSEPs remained reduced from baseline. The operation was completed and the patient awoke with weakness and incoordination of her lower extremities. During the first few months after the operation, the patient’s strength and coordination improved, but she continues to struggle with ambulation at a follow-up period of 36 months.
Team Notes
The pre-positioning baseline traces are represented in Fig. 28.2a. Figure 28.2b represents tracings 1 h after the baseline traces were recorded, coinciding with the patient having just been placed in the prone position, neck flexed with the head held in a fixation device. During surgery, an amplitude decrease and a latency shift in the tracings should prompt a differential diagnosis including excessive surgical retraction, hypotension, ischemia, or positioning injury. After the operation, a closer review revealed increasing latency and a diminution in amplitude of the SSEP traces , particularly pronounced in the lower extremity SSEPs, shortly after initial positioning (i.e., after flexing the neck). These circumstances should have immediately raised suspicion of a positioning-related problem. The events of this particular case provide a strong argument for prepositioning baseline neurophysiologic testing as well as postpositioning testing. Baseline traces become invaluable when the practitioner is confronted with patients with neuropathy caused by a comorbid condition such as diabetes mellitus or elderly patients with fewer active nerve fibers and, hence, SSEPs of potentially lesser amplitude. In the case of this 28-year-old woman undergoing an operation for Chiari malformation, detecting the SSEP changes after initial positioning would have allowed for early correction and adjustment of the surgical position.
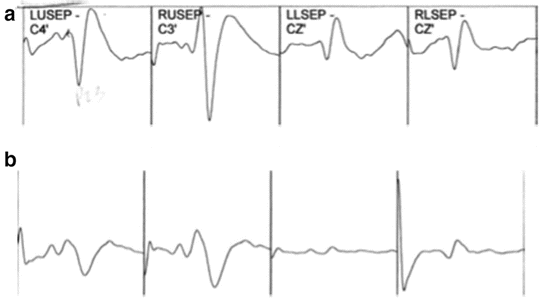

Fig. 28.2
Pre-positioning baseline SSEP traces (a). SSEP traces 1 h after positioning (b)
In principle, both the surgeon and anesthesiologist should agree on the amount of neck flexion acceptable for surgical exposure as well as adequacy of ventilation and adequacy of venous outflow of the head and neck to prevent macroglossia and intracranial hypertension. When possible, two to three fingerbreadths of space between the anterior mandible and the sternal notch is suggested. In this case, the patient’s neck was likely overflexed, which led to chronic stretching and vasospasm of the posterior spinal radicular arteries with presumed diminution in perfusion. Vigilance and attention to monitoring traces are important at all stages of the operation in order to detect a correctable insult early.
To review the information on somatosensory-evoked potentials from Chap. 1, stimulation of the median nerve leads to an evoked potential at the brachial plexus. This P9 response is recorded at Erb’s point (slightly above the midportion of the clavicle) and is generated from the nerves of the brachial plexus near the point at which they enter the spine. The recordings at Erb’s point indicate brachial plexus activity and confirm appropriate electrical stimulation of the median nerve. Prolongation of the interval between the P14 and the N20 peaks is referred to as the central conduction time. This interval indicates changes in the integrity of the primary somatosensory system. Thus, a prolonged central conduction time is a sign of ischemia. Recall the P14–P16 waves are generated in close proximity to the dorsal column nuclei while the N20 wave is generated in the primary somatosensory cortex. Whereas the conduction time in the median nerve may be increased in a cool upper extremity during a lengthy surgical procedure, central conduction time is not affected. Factors affecting central conduction time include surgical retraction, anesthetic depth, hypothermia (drop in core temperature), and hypotension—the last three of which affect SSEPs bilaterally.
A comprehensive differential diagnosis for decreased amplitude of SSEPs along with a latency shift should include consideration of physiologic factors, technical causes, responses to anesthetic drugs, surgical events, and patient positioning. Physiologic factors such as hypotension or a decrease in core temperature can cause changes in the SSEPs. Timely assessment of the patient’s vital signs can confirm the absence of hypotension contributing to ischemia or a decrease in body temperature. Hypotension, in general, leads to a more global decrease in amplitude, whereas hypothermia causes a slowing of the neural conduction velocity in peripheral nerves and a gradual increase in the latency of the SSEPs [18]. Mean arterial blood pressure should be increased if a hypotensive or hypoperfusion insult is suspected. Upon discovery of hypoperfusion in the spinal radicular arteries in the case above, direct application of papaverine was used in addition to pressor medications. Interestingly, the use of papaverine hydrochloride as a vasodilator to treat vasospasm has been implicated in transient cranial nerve dysfunction. Its application has been reported to affect the oculomotor and facial nerves, as well as cause auditory dysfunction [19].
Technical causes for response changes can sometimes be detected by increases in electrode impedance. Electrode placement, optimal stimulus intensity, and stimulus rate are important factors that can affect the measured responses during any operation. Such issues as sweat and oil on a patient’s skin can lead to changes in contact impedance if surface electrodes are utilized. Environmental (electrical) interference can also influence response waveform morphology.
Anesthetic drugs, particularly the halogenated agents, can produce a dose-related increase in latency and decrease in amplitude of SSEP tracings. Although SSEPs are not as sensitive to such changes as are motor-evoked potentials, it is best to maintain a steady state of anesthesia and avoid giving a bolus of a drug. Nitrous oxide can also increase the latency and decrease the amplitude of cortical SSEPs. In the above case, nitrous oxide was avoided and anesthesia was maintained with sevoflurane, propofol, and remifentanil infusions. Sevoflurane remained constant at 0.5 minimum alveolar concentration (MAC) in order to minimize drug-induced SSEP changes. Although halogenated inhalational agents produce a dose-related increase in latency and decrease in amplitude of the SSEPs, intravenous agents have minimal impact on SSEPs at low to moderate doses. (For more information, see Chap. 19, “General Anesthesia for Monitoring”.)
In a suboccipital decompression surgery for Chiari malformation, during the resection of the cerebellar tonsils, it is not uncommon to see “noise” on the SSEP tracings (Fig. 28.3). After the “noise” has resolved, it is imperative to establish whether or not there has been any change from baseline. If a change in SSEPs persists, the team must perform a timely analysis of the problem, taking into account the aforementioned variables.