Chapter 71
Stroke 
Definitions and Classification of Strokes
Stroke Mimics
Other neurologic and systemic conditions can cause abrupt neurologic deficits that may be difficult to distinguish from acute stroke. Migraine headaches can be associated with transient focal neurologic symptoms preceding or during the early phase of the headache. Rarely, these deficits may persist and result in frank infarction. Focal seizures may manifest with deficits such as aphasia, focal weakness, or sensory symptoms that can mimic a stroke. In addition, postictal neurologic deficits (Todd’s paralysis) may persist for greater than 24 hours after a seizure. Finally, global metabolic stress (for example, marked hyperglycemia, acidosis, or electrolyte disturbance) can cause focal neurologic deficits. This likely occurs because the metabolic stress unmasks preexisting focal neurologic damage (such as a prior stroke from which the patient has recovered).
Initial Diagnosis and Management
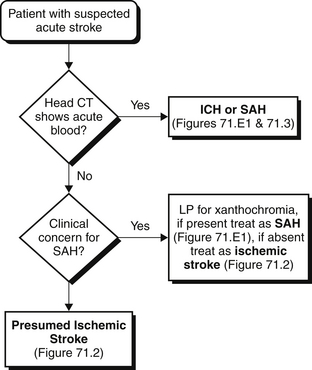
The initial diagnosis and management of stroke patients typically occur in the emergency department. Because different stroke types have markedly divergent management strategies and potential acute treatments (such as thrombolysis using intravenous [IV] tissue plasminogen activator, described later) that can only be given within a short time window, a directed approach is required when evaluating patients who present with acute neurologic deficits (Figures 71.1 to 71.3). A clear history of the time of symptom onset is needed to determine whether the patient may be eligible for thrombolysis. If the patient or family members cannot provide an exact time when symptoms occurred, the time when the patient was last seen normal is assumed to be the time of symptom onset. Certain historical features may help distinguish stroke subtypes. For example, sudden-onset “thunderclap” headache, neck stiffness, and nausea that coincide with neurologic symptom onset strongly suggest SAH (although headache is often nonspecific and does not reliably distinguish ischemic from hemorrhagic stroke). In contrast, symptoms that develop over minutes are more likely to be associated with ICH.
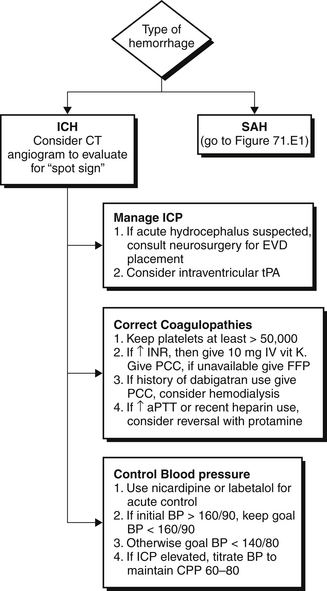
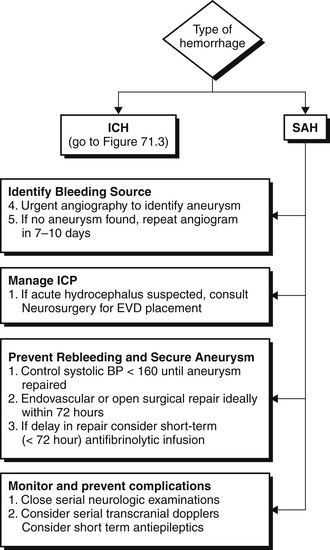
After a clinical diagnosis of acute stroke has been made, it is imperative to distinguish hemorrhagic from ischemic stroke as rapidly as possible and initiate appropriate therapy. A computed tomographic (CT) scan is usually the first neuroimaging study to be performed because it is readily available and highly sensitive for acute bleeds (see Figures 71.1 to 71.3 and Figure 71.E1) ![]() . Intraparenchymal or intraventricular hyperdense lesions suggest ICH, whereas hyperdensities within the sulci and basal cisterns imply SAH. In the absence of evidence for hemorrhage, an ischemic infarct is presumed because radiologic signs of cerebral ischemia may be subtle or undetectable within the first 12 hours. It is important to realize, however, that CT can miss a small proportion of SAH (< 5%). Thus, if clinical suspicion for SAH is high, a negative CT should prompt further testing with lumbar puncture to look for red blood cells or xanthochromia (yellowish discoloration of CSF) or with a magnetic resonance imaging (MRI) scan.
. Intraparenchymal or intraventricular hyperdense lesions suggest ICH, whereas hyperdensities within the sulci and basal cisterns imply SAH. In the absence of evidence for hemorrhage, an ischemic infarct is presumed because radiologic signs of cerebral ischemia may be subtle or undetectable within the first 12 hours. It is important to realize, however, that CT can miss a small proportion of SAH (< 5%). Thus, if clinical suspicion for SAH is high, a negative CT should prompt further testing with lumbar puncture to look for red blood cells or xanthochromia (yellowish discoloration of CSF) or with a magnetic resonance imaging (MRI) scan.
Management of Ischemic Stroke
Acute Reperfusion Strategies
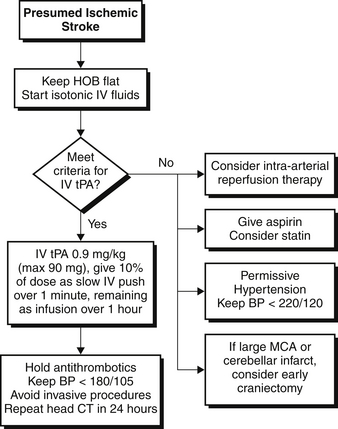
Multiple double-blinded randomized controlled trials have shown that thrombolysis and reperfusion using alteplase, also called tissue plasminogen activator (tPA), can significantly improve outcome when given early to patients with ischemic stroke (Figure 71.2). In these trials, patients treated with IV tPA within 3 hours of symptom onset had significant improvement in neurologic outcome at 3 months. These benefits were independent of the type of ischemic stroke. Subsequent trials and new guidelines extended the time window for tPA usage to within 4.5 hours of symptom onset.
Supportive Therapy
Most patients who present with ischemic stroke unfortunately are unable to receive acute thrombolysis because of the narrow time window and other selection criteria. In these patients, care is directed at aggressively preventing stroke progression and limiting other medical complications (see Figure 71.2). The occlusion of a cerebral artery produces a core of nonviable brain tissue that is surrounded by a zone of viable but at-risk tissue known as the penumbra. Maximizing blood flow to the penumbra while limiting the core infarct area is a central principle of medical management of ischemic stroke.
Antiplatelet agents such as aspirin provide a small but significant mortality benefit and decrease acute recurrent stroke risk and should be administered early (within 24 hours) after ischemic stroke onset. Statins may also have an acute benefit through unclear mechanisms (possibly by limiting the extent of inflammatory injury). Systemic anticoagulation (for example, with IV unfractionated heparin) should in general be avoided, as studies suggest that the risk of hemorrhagic transformation outweighs the benefit of recurrent stroke prevention in the first few days after stroke onset. Anticoagulation may be useful in certain circumstances, for example, in patients with fluctuating symptoms (implying an unstable thrombus) or in the setting of arterial dissection, but these areas remain highly controversial.
Similar to fever, multiple studies showed a relationship between admission hyperglycemia and worsened neurologic outcome. A multicenter randomized controlled trial of intensive glycemic management in ischemic stroke patients is currently under way. In the meantime, it is reasonable to control blood sugar in a similar fashion to other critically ill medical patients (see Chapter 12).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree