INTRODUCTION
Although nontraumatic subarachnoid and intracerebral hemorrhages account for a relatively small portion of ED visits, a missed diagnosis can produce devastating results. Early recognition and aggressive management may improve outcomes.
Subarachnoid hemorrhage is the leakage of blood into the subarachnoid space, most often due to a ruptured intracranial aneurysm. The classic presentation is a sudden, severe headache.
Intracerebral hemorrhage, or hemorrhagic stroke, typically presents as an acute neurologic deficit, often accompanied by headache. The features and treatment of subarachnoid and intracerebral hemorrhage are discussed in this chapter. Management of intracerebral hemorrhage is very different from the management of ischemic stroke. Ischemic stroke is discussed in chapter 167, Stroke Syndromes.
SUBARACHNOID HEMORRHAGE
About 75% of subarachnoid hemorrhages are caused by a ruptured aneurysm. In about 20%, a cause is not identified.1 The remaining causes are related to a variety of miscellaneous conditions, including arteriovenous malformations, sympathomimetic drugs, and other less common causes. About 20% of patients with one aneurysm will have an additional aneurysm, which makes identification of the initial aneurysm important.
Two percent of family members of patients with subarachnoid hemorrhage will develop the same disease. This risk rises with increasing number of family members involved or with a family history of adult polycystic kidney disease.1 Hypertension and smoking increase the risk. Additional risk factors are listed in Table 166-1.
Cerebral aneurysms are focal arterial pouches typically located in areas of bifurcation of the circle of Willis. While the precise pathophysiology is not known, many factors have been associated with aneurysmal development and rupture. Such factors include familial/genetic predisposition, cellular aberrations in vascular wall repair or remodeling, and aberrations in local blood flow.2 While it is not possible to predict rupture risk of a particular aneurysm, larger aneurysms (>5–10 mm) are more likely to rupture than smaller aneurysms.2,3
Patients with subarachnoid hemorrhage classically present to the ED with a severe headache of acute onset (termed a “thunderclap” headache) that reaches maximal intensity within minutes. Typically, the headache persists for several days, but may resolve in a shorter period.1 Subarachnoid hemorrhage is diagnosed in 11% to 25% of patients who present to the ED with a thunderclap headache.4,5 Even if a patient is not experiencing the “worst ever” headache, a headache that is different in intensity or quality from past headaches raises concern for subarachnoid hemorrhage. Headaches associated with loss of consciousness, seizure, diplopia or other neurologic signs, or nuchal rigidity also require clinical investigation.6 Less frequently, patients may present with nausea and vomiting, altered mental status, photophobia, or symptoms suggestive of ischemic stroke. Approximately 20% of patients develop their symptoms while engaged in activities that cause increased blood pressure, such as exercise, sexual intercourse, or defecation. Isolated, uncomplicated true syncope without head trauma, headache, seizure, neurologic deficits, nuchal rigidity, or other symptoms of subarachnoid hemorrhage does not require evaluation for subarachnoid hemorrhage. In the absence of blunt trauma, subhyaloid retinal hemorrhage is pathognomonic of subarachnoid hemorrhage but is not commonly seen.
Patients with subarachnoid hemorrhage who are misdiagnosed at their initial ED visit have worse outcomes than those who are diagnosed early.7,8 Misdiagnosis is associated with normal mental status (present in about half of patients with subarachnoid hemorrhage) and smaller size of hemorrhage.8 Complications of missed diagnosis include repeat hemorrhage and obstructive hydrocephalus. Symptomatic improvement following analgesics does not exclude life-threatening causes of headache.9 Table 166-2 lists the differential diagnosis of subarachnoid hemorrhage.
Vascular (other intracranial hemorrhage, ischemic stroke or transient ischemic attack, arterial dissection, venous thrombosis) Drug toxicity Infection (meningitis, encephalitis) Intracranial tumor Intracranial hypotension Metabolic derangements Primary headache syndromes (benign thunderclap headache, migraine, cluster headache) Hypertensive disorders |
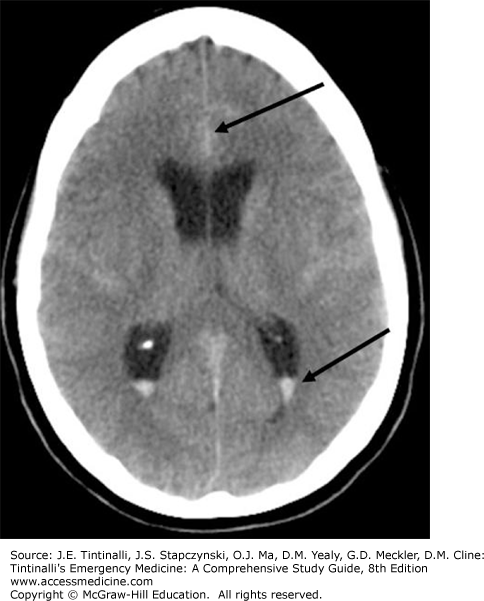
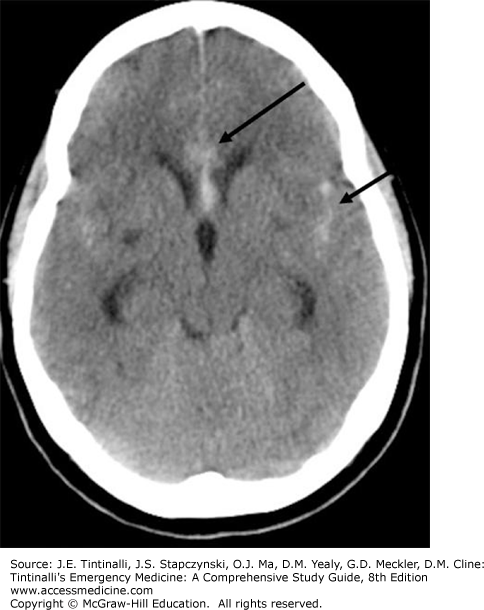
The initial diagnostic modality of choice when subarachnoid hemorrhage is suspected is a noncontrast CT of the head (Figures 166-1 and 166-2). The sensitivity of CT in diagnosing subarachnoid hemorrhage is highest shortly after symptoms begin and is estimated to be 98% within 6 to 12 hours of the onset of symptoms. Sensitivity decreases to about 91% to 93% at 24 hours and continues to decline rapidly thereafter, reaching 50% at 1 week.6,9 Newer-generation CT scanners provide increased sensitivity for detecting subarachnoid hemorrhage, especially in the setting of (1) patients presenting within 6 hours of symptom onset, and (2) greater availability of a timely interpretation by a neuroradiologist.10,11,12 For suspected subarachnoid hemorrhage, a negative head CT is typically followed by LP (see later discussion).
FIGURE 166-1.
Diffuse subarachnoid hemorrhage with associated ventricular hemorrhage. Top arrow indicates blood in interhemispheric fissure. Bottom arrow indicates blood in lateral ventricle. [Image used with permission of James Anderson, MD, Department of Radiology, Oregon Health & Science University.]
CT/CT angiography (CTA) and MRI/MRA are options after a negative head CT, when these studies are clinically appropriate and available.13 In a small study, two of 116 patients had an aneurysm discovered by CTA after normal findings on both CT and LP.14 The probability of excluding a subarachnoid hemorrhage following CT/CTA is about 99.4%.15 Important consequences of this diagnostic pathway include the detection of incidental aneurysms, as opposed to clinically significant bleeds, with the background incidence of aneurysms in the population (2% to 6%) exceeding that of the morbidity and mortality associated with subarachnoid hemorrhages.16 The major disadvantage of CT/CTA is ionizing radiation. The usefulness of MRI, particularly fluid-attenuated inversion recovery MRI sequences, is limited.17 A negative MRI result would still need to be followed by an LP.18 The major disadvantages of MRI/MRA at this time are availability, time to perform the examination, and cost.
Most authorities recommend CSF analysis when a patient with suspected subarachnoid hemorrhage has a normal result on head CT.19,20 Another advantage of LP is the ability to identify other causes of headache such as meningitis or idiopathic intracranial hypertension. The disadvantages of LP include post-LP headache and inability to perform the procedure in the patient with coagulopathy or thrombocytopenia.
The two CSF tests of greatest interest are the presence of xanthochromia and RBC count. Xanthochromia is a yellow appearance of the CSF due to the enzymatic breakdown of blood releasing bilirubin. Any exposure of the CSF to light prior to interpretation can increase the rate of bilirubin degradation, which decreases any xanthochromia present.1 Similarly, a delay in processing the CSF specimen may result in the development of xanthochromia following a traumatic LP. CSF is evaluated for xanthochromia with visual inspection, the standard technique in most U.S. laboratories, or by spectrophotometry, which may have superior sensitivity but approximately 75% specificity, resulting in additional unnecessary diagnostics for false positives.21 The utility of the test is further limited in that it takes approximately 12 hours for xanthochromia to develop in CSF.22
The RBC count in the third or fourth tube of CSF is commonly used to identify subarachnoid hemorrhage. Several issues can make interpreting CSF results challenging. The number of RBCs that constitutes a “positive” LP result has never been clearly defined, nor has the number of RBCs that may be attributed to a “traumatic” LP. One study showed that approximately 10% or 15% of LPs are traumatic, using cutoffs of 400 and 1000 RBCs, respectively.23 A comparison of cell counts between consecutive tubes or between tubes 1 and 4 is sometimes used to differentiate subarachnoid hemorrhage from a traumatic LP. A small study, however, demonstrated that a 25% reduction in RBCs between tubes 1 and 4 may occur even in cases of confirmed subarachnoid hemorrhage.24 Another small study found that RBCs <100 in the final tube effectively ruled out subarachnoid hemorrhage, whereas an RBC count of >10,000 in the final tube was associated with an increase in the odds of subarachnoid hemorrhage by a factor of 6.25
In general, normal findings on head CT, the absence of xanthochromia, and zero or few RBCs (<5 × 106 RBCs/L) in the CSF help reliably exclude subarachnoid hemorrhage.26 A normal head CT result with a positive finding of xanthochromia or elevated RBC count should be considered diagnostic of subarachnoid hemorrhage. Unfortunately, the literature remains unclear on the precise threshold number of RBCs needed in the CSF to be considered diagnostic of subarachnoid hemorrhage.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree