
Spinal Cord Injuries
Brian D. Mahoney
The clinical spectrum of spinal cord injury varies from the awake patient complaining of pain, paresthesias, numbness, weakness, or paralysis to the unconscious patient (usually due to associated head injury) unable to give any clues about the severity of his or her injuries. In the United States, most spinal cord injuries are due to motor vehicle collisions (37%), followed by falls (29%), violence (14%), sports and recreational activities (9%), and other causes (11%) (10). Males comprise 81% of these patients with an average age of injury of 43 (10). In the United States, the annual incidence of spinal cord injuries is approximately 40 per million population (10). Many patients with spinal cord injury die soon after their injury because of either high cord injury or other associated traumatic injuries. Life expectancy is significantly below that of patients without spinal cord injury (10). Of those who survive their initial injury and hospitalization, illnesses with the greatest impact on life expectancy are pneumonia and sepsis (10). Direct lifetime expenses for a 25-year-old patient at the age of injury with high quadriplegia exceeds $4.5 million (10).
Normally, the spinal column ensures stability and protects the cord, the spinal roots, and the cauda equina from injury. Panjabi and White (11) defined spinal stability as “the ability of the spine, under physiologic loads, to maintain the relationships between vertebrae such as there is neither damage nor irritation to the spinal cord or roots, nor incapacitating deformity nor pain due to structural changes.” Injury can render the usually protective spinal column a threat to the cord and its roots. The cord can suffer injury through compression, crush, laceration, penetration, stretch, inflammation, infection, hypoxia, or ischemia. In unusual circumstances, the spinal column can be rendered unstable by infectious or inflammatory causes, such as atlantoaxial dislocation in patients with rheumatoid arthritis or inflammation associated with pharyngeal infections.
The most mobile segments of the spinal column (C4, C5, C6, L1, L2) place the adjacent segments of the spinal cord at higher risk for injury. Spinal cord injury at discharge is as follows (10): incomplete quadriplegia 41%, incomplete paraplegia 19%, complete paraplegia 18%, complete quadriplegia 12%, complete neurologic recovery less than 1%.
The ribs buttress the thoracic spinal column, providing a relatively safe environment for the cord. However, when there is thoracic bony displacement or soft tissue swelling, the cord injury is usually devastating. The spinal canal is relatively narrow here, and the thoracic cord is an area of watershed circulation. At L2, the spinal cord ends and the spinal canal is filled with the cauda equina—a collection of spinal nerves passing from the cord to their respective lumbar and sacral foramina. Though certainly at risk for acute cauda equina syndrome, these peripheral nerves are relatively resistant to injury compared to the spinal cord.
CLINICAL PRESENTATION
The patient may describe pain or tenderness at the level of injury and weakness, numbness, or paresthesias distal to the injury level. Paresthesias at the time of injury are a sign that the cord or roots have been compressed. Late paresthesias may be a sign of instability of the spinal column and should lead to a comprehensive search for instability. History and physical examination establish motor (eFig. 30.1), reflex, or sensory deficits (eFig. 30.2 and eFig. 30.3), as well as fecal incontinence or urinary retention. Urinary retention may present as oliguria with overflow stress incontinence. Careful mapping of the level of sensory loss (including attention to areas around the anus for lower segmental reflexes and for sacral sparing) identifies the level of cord injury and the level at which radiologic studies should be focused (Fig. 30.1).
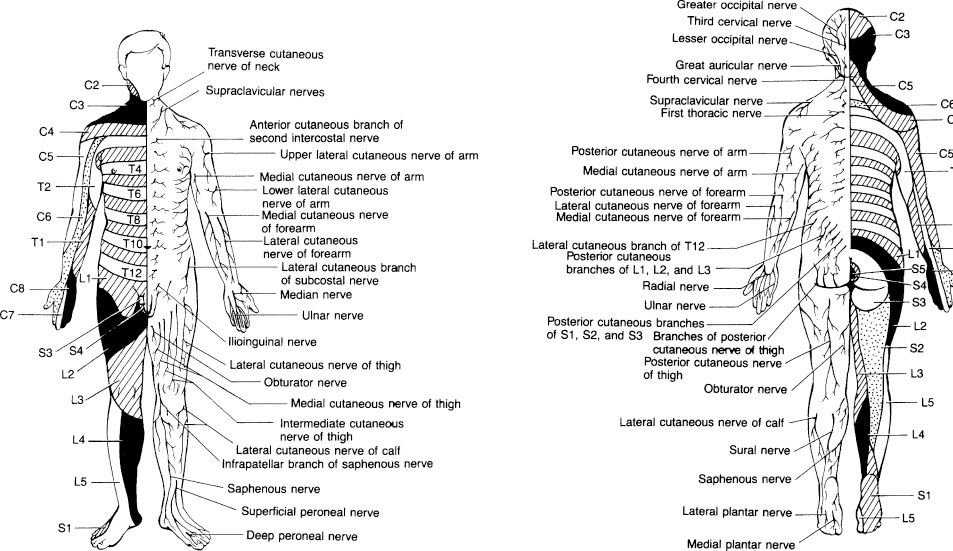
FIGURE 30.1 Sensory dermatomal segments: anterior view (left) and posterior view (right). IDAMS Asset Name: 4766.101.001.tif Credits. (From Harwood-Nuss A, Wolfson, AB, Linden CH. The Clinical Practice of Emergency Medicine. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001.)
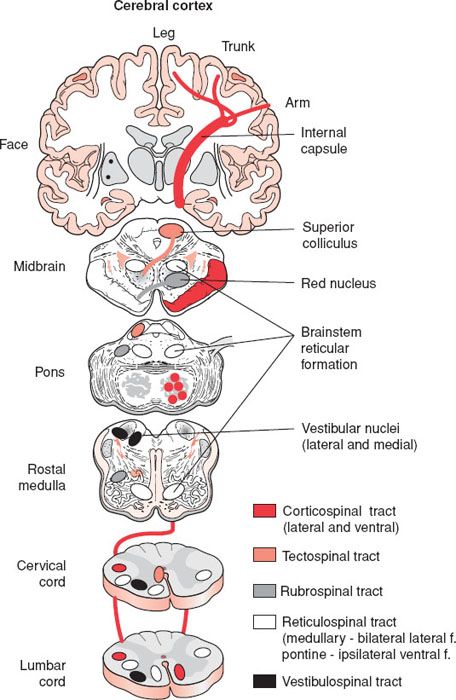
eFIGURE 30.1 A diagrammatic representation of the main descending motor pathways connecting the brain to the spinal cord for one side of the body. Approximately 90% of the corticospinal axons cross at the spinomedullary junction and comprise the lateral corticospinal tract. The remaining 10% form the ipsilateral ventral corticospinal tract. Axons from cells in the contralateral superior colliculus pass through the brainstem and enter cervical levels of the spinal cord in the tectospinal tract. The red nucleus sends axons to the contralateral spinal cord in the rubrospinal tract. Neurons from many levels and both sides of the reticular formation form the reticulospinal tract. The medial and lateral vestibular nuclei send axons to the spinal cord in the vestibulospinal tract. This is mainly and ipsilaterally projecting pathway. (From Schwartz ED, Flander AE. Spinal Trauma: Imaging, Diagnosis, and Management. Philadelphia, PA: Lippincott Williams & Wilkins; 2007.)

eFIGURE 30.2 The transmission of pain from the body to the brain occurs in the spinothalamic tract. Axons of the anterolateral system terminate in the several regions including the reticular formation throughout the brainstem, superior colliculus, periaqueductal gray, and several thalamic nuclei.
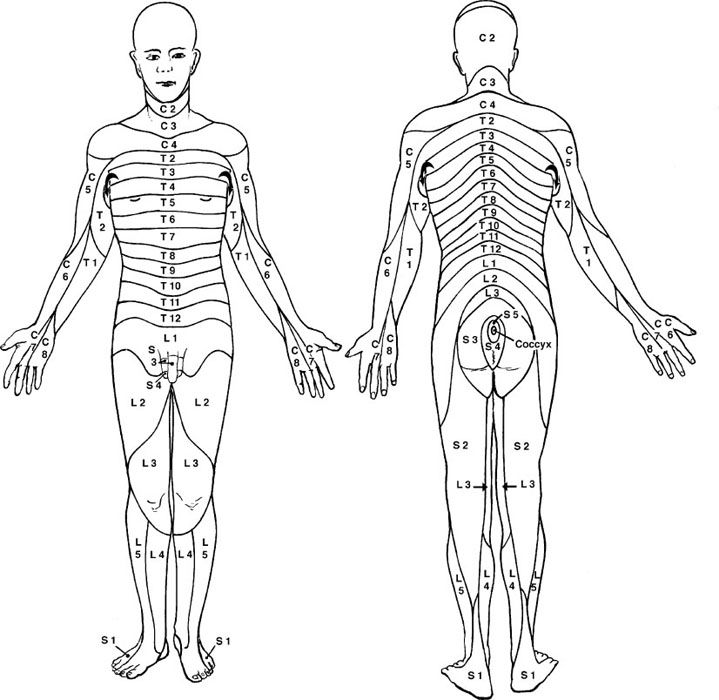
eFIGURE 30.3 Dermatomes represent the cutaneous distribution of territories innervated by spinal nerves. (From Schwartz ED, Flander AE. Spinal Trauma: Imaging, Diagnosis, and Management. Philadelphia, PA: Lippincott Williams & Wilkins; 2007.)
When the patient has no function of any segment of the cord below the level of injury, the injury is physiologically complete. Such a circumstance carries with it a grim but not hopeless prognosis for meaningful recovery. It is nearly impossible to accurately diagnose a permanent physiologically complete cord injury in the emergency department (ED) because spinal shock may be a concurrent factor. Spinal shock is the temporary loss of spinal reflex activity that accompanies the motor and sensory losses. Males may exhibit priapism. Ditunno et al. (4) describe four phases of spinal shock lasting a variable amount of time, from a day to weeks.
• Phase 1: Flaccid paralysis, areflexic, lasts about 1 day.
• Phase 2: Return of polysynaptic reflexes, e.g., bulbocavernosus, 2 to 3 days.
• Phase 3: Return of monosynaptic deep tendon reflexes.
• Phase 4: Hyperreflexic spastic paralysis (eFig. 30.4).
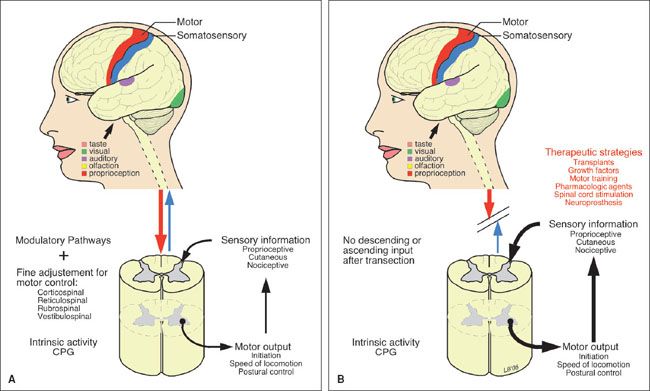
eFIGURE 30.4 Spinal transection creates a new spinal cord. Spinal transection results in considerable modification of the circuitry caudal to the lesion. Some of these changes may contribute to the deficits, while others may provide opportunities for therapeutic interventions. A: The intact spinal cord receives control and modulation from supraspinal systems and sensory input from primary afferents, which determines the activity of spinal circuits, including the central pattern generator. B: After SCI, descending pathways are lost, their denervated receptors up regulate, and afferent pathways are enhanced, leading to hyperexcitable reflexes and spastic paralysis. Strategies targeting independent mechanisms are the bases of potential treatments. (From Schwartz ED, Flander AE. Spinal Trauma: Imaging, Diagnosis, and Management. Philadelphia, PA: Lippincott Williams & Wilkins; 2007.)
The bulbocavernosus reflex is elicited when squeezing or tugging on the glans penis causes reflex contraction of the anal sphincter. Some patients who initially appear physiologically completely paralyzed and have spinal shock may go on to remarkable recoveries after the initial phases of spinal shock have passed.
The patient with a cervical or high thoracic cord injury above T6 may present with relative hypotension due to the sudden loss of sympathetic tone below the level of the lesion (eFig. 30.5). The patient has warm, dry skin; normal capillary refill; and a paradoxical bradycardia. Labeling this condition spinal neurogenic shock (eFig. 30.6) helps to differentiate it from the aforementioned spinal shock. Spinal neurogenic shock in the patient with multiple injuries may be difficult to differentiate from other causes of shock. Hemorrhagic shock classically presents as hypotension, tachycardia, cool and moist skin, and delayed capillary refill. The hypotensive patient should be assumed to have hemorrhagic shock until all external bleeding is controlled and the search for occult hemorrhage in the chest, abdomen, retroperitoneum, and pelvis is complete. This search should include, at a minimum, chest x-ray, FAST cardiac and abdominal ultrasonography, urinalysis, and pelvic x-ray or abdominal and pelvic computed tomography (CT). After this survey is complete and no other explanation for shock is found, the hypotension may be attributed to spinal neurogenic shock. At that point, after adequate initial volume resuscitation of the vasodilated patient, fluid administration should be kept at maintenance levels and the hypotension should be treated with pressors as necessary to maintain perfusion of the brain and spinal cord. Excessive fluids may cause pulmonary edema and contribute to worsening spinal cord edema. Ischemia is a significant contributor to the ultimate degree of spinal cord injury. Unfortunately, there are no prospective controlled human studies to determine what the optimal blood pressure is to maintain spinal cord perfusion and thus limit secondary injury to cord tissue. Typically, the patient is maintained at a blood pressure sufficient to sustain intact mentation and urinary output. Animal studies show that hypotension worsens neurologic outcome. For this reason it is recommended that systolic blood pressure below 90 mm Hg be avoided or corrected as soon as possible and that the mean arterial pressure be maintained at 85 to 90 mm Hg for the first 7 days (13).
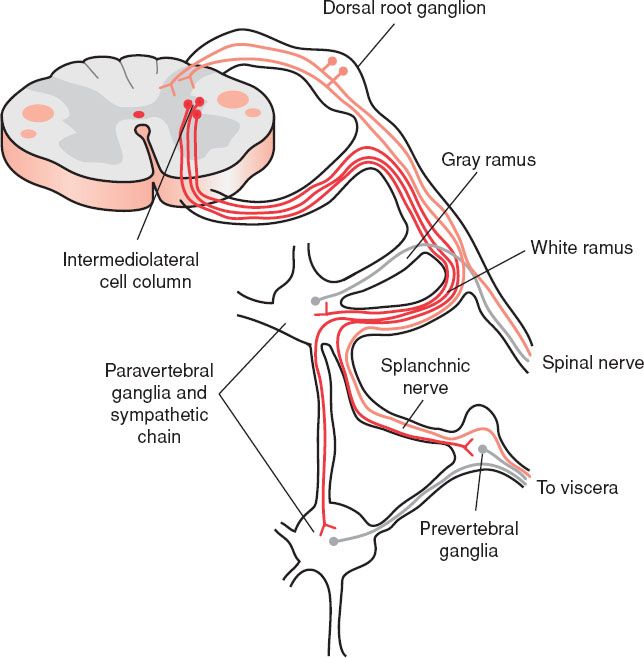
eFIGURE 30.5 The sympathetic nervous system and its relationship to the central nervous system. Sympathetic preganglionic neurons (red) are located in the intermediolateral cell column of the thoracic spinal cord. Their axons leave the spinal cord via the ventral root and enter paravertebral and sympathetic chain ganglia on the ipsilateral side of the body through the white communicating ramus. Some fibers synapse within ganglia at the same segmental level, others travel up and down the sympathetic chain to other ganglia. Postganglionic sympathetic fibers arising from cells in the paravertebral or sympathetic chain ganglia (green) join a spinal nerve by passing through a gray communicating ramus. These supply smooth muscle and glands of the body wall. Other postganglionic fibers travel through a splanchnic nerve to innervate viscera of the body cavities. Axons from sensory (pain) neurons (blue) supplying an internal organ may pass through autonomic ganglia, but they originate from neurons in the dorsal root ganglia. (From Schwartz ED, Flander AE. Spinal Trauma: Imaging, Diagnosis, and Management. Philadelphia, PA: Lippincott Williams & Wilkins; 2007.)
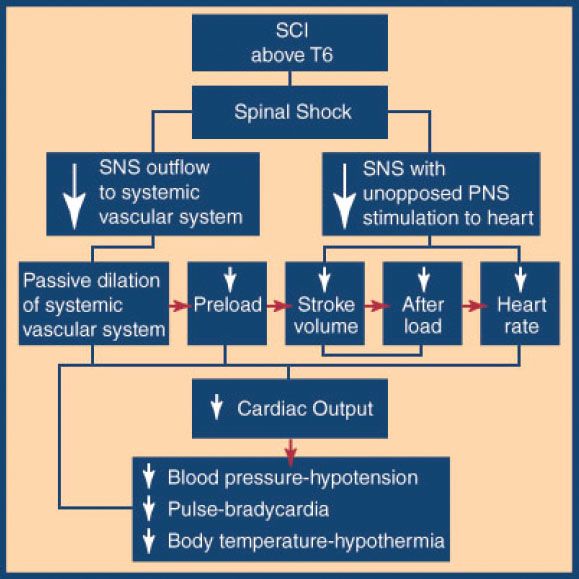
eFIGURE 30.6 Spinal neurogenic shock. Spinal cord injury above T6 impairs sympathetic nervous system activity caudal to injury and leaves unopposed parasympathetic nervous system activity.
In the past 20 years, the percentage of patients with complete spinal cord injuries has fallen while incomplete spinal cord lesions have increased. This has been attributed to improved management at the scene and in the hospital. There are several important partial cord syndromes (Fig. 30.2).
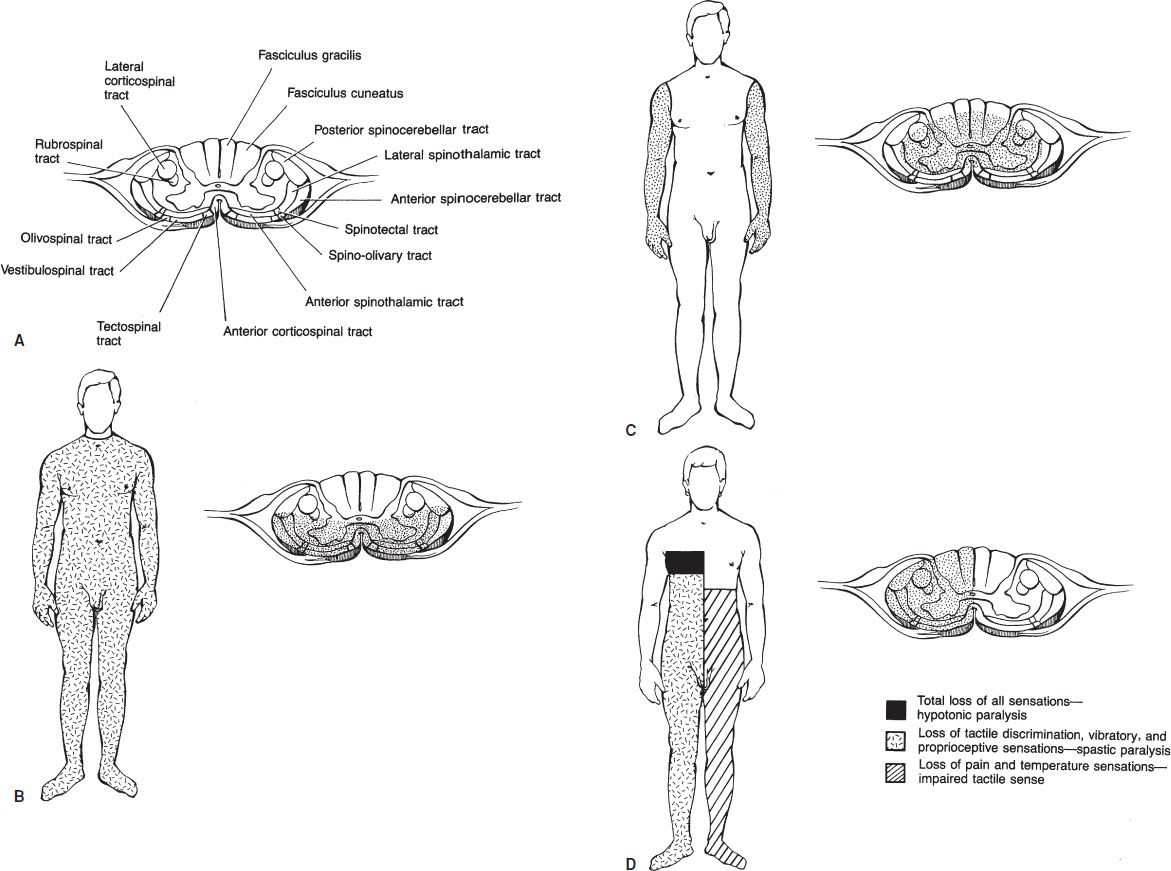
FIGURE 30.2 Transverse section of the spinal cord at the cervical level: the ascending and descending tracts (A), tracts that may be damaged in the anterior spinal artery syndrome (B), tracts that may be damaged in the central cord syndrome (C), involved tracts and the superimposed areas of deficit from an injury at T4 (D), resulting in the Brown-Séquard syndrome.









