Fig. 31.1
Example of basic wet lab. This operating microscope has a second ocular for a second person to observe
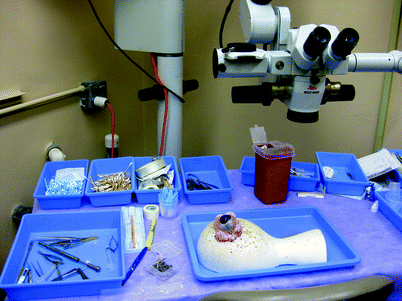
Fig. 31.2
View of wet lab setup with a porcine eye. It is evident that the porcine eye is much larger than the human eye
Lee et al. outlined the structured wet lab program developed at the University of Iowa [15]. In this program, a set of goals and objectives is established for trainees. This allows assessments tied to the objectives to ensure that the learner is ready for the next step. The program proposes a set of practical and didactic tests to ensure competence. This allows residents to learn skills at the appropriate time and provides a more reliable skill set prior to the resident’s first real case in the operating room. One of the most important features and logistically challenging components of this program is having faculty available for significant portions of the wet lab training, which at best would occur during normal hours and not late at night or on weekends when faculty are less available (see Table 31.1).
Table 31.1
Summary of First-Year Resident Iowa Wet Laboratory Curriculum
Objectives: During the first-year resident’s rotation at a given center, the resident will have 5 half-day sessions in the wet laboratory under faculty supervision. Additional practice will be required and this is the responsibility of the resident. The resident is also required to read Cataract Surgery for Greenhorns by Oetting and Phacodynamics by Seibel prior to the first wet lab session: |
1. Demonstrate fine motor and proprioception kills under the operating microscope. |
2. Demonstrate proficiency in working in small surgical field alone and with an assistant. |
3. Demonstrate knowledge of the various phacoemulsification machines and settings of each machine. |
4. Demonstrate phacoemulsification machine and operating microscope pedal functions. |
5. Demonstrate performance of five clear corneal and scleral incisions on animal eye. |
6. Identify the steps of phacoemulsification. |
7. Demonstrate the performance of the steps of phacoemulsification on animal eye. |
8. Identify the various types of ophthalmic sutures. |
9. Demonstrate placing corneal, scleral, conjunctival, and skin sutures. |
Fisher outlined a structured skills “obstacle course” wet lab with specific stations to assess residents’ skills on a variety of surgical tasks [16]. The “obstacle course” was designed to simulate three surgical procedures: (1) temporal artery biopsy and skin suturing, (2) muscle recession, and (3) phacoemulsification wound construction and suturing. All the procedures were performed in a wet lab using a pig foot and eye. The pig’s foot was prepared for the temporal artery biopsy and skin suturing section by inserting a piece of red plastic tubing into the superficial facial to simulate the artery. At each station the resident was given a list detailing the required steps of the test and videotaped at each station. The videos were later reviewed by various experienced surgeons and given a score. The authors conclude that this wet lab obstacle course demonstrated development of an objective and standardized test of beginning surgical skills and established face and content validity for ophthalmic surgery [16].
Henderson et al. outlined the key components of an effective ophthalmic wet lab: setting up the physical space, establishing appropriate faculty and curriculum, obtaining the practice eye, stabilizing the eye, preparing the eye, and funding the wet lab [11]. Having a separate and dedicated space for the wet lab allows the trainee to practice when appropriate and when faculty and the resident are available. As mentioned above, a structured program is a key to a successful wet lab program [11, 15]. Practice eyes are sometimes expensive or hard to obtain. Typically, porcine eyes are used but grapes, sheep eyes, cadaver eyes, cadaver eyes with glued-on contact lenses, and plastic-fabricated eyes can be used for simulating the live human eye in the wet lab [15, 17–20]. Various techniques have been outlined to make the porcine eye behave more like the senile human eye, such as the application of fixative solutions and microwave energy [11]. Each of these techniques has its advantage in some capacity in attempting to simulate a human eye; however, no single technique or model is as good of a replacement as operating on a live, human eye. One of the most difficult issues of establishing a wet lab, however, is simply funding the lab which requires space, instruments, and most importantly faculty time. Still, few simulation modalities are as tried and true as the wet lab, and as such, this classic simulation has staying power.
Computer-Based Simulation
Advances in computer technology have led to increasingly sophisticated virtual reality simulators for surgical and procedural preparation. As outlined in this text, simulation training has been gaining popularity among many surgical subspecialties and in the education of teaching procedural tasks to nonsurgical residents. In ophthalmology, there are currently two commercially available ophthalmic simulators: PhacoVision® (Melerit Medical; http://www.meleritmedical.com/) and Eyesi® (VRmagic Holding AG; http://www.vrmagic.com/en/eyesi/) (Figs. 31.3, 31.4, 31.5, and 31.6). These devices are primarily aimed at cataract surgery; however, the initial versions of the Eyesi® simulator were designed to simulate vitreoretinal surgery [21–25]. Although both simulators have many similarities and functions, our discussion will focus on the Eyesi® simulator since it is the most widely studied device of the two [24, 26–29].
There are significant advantages in the use of virtual reality ophthalmologic simulators for training. The computer-based simulators such as the Eyesi® are cleaner than wet labs, allow for quick setup of simulation exercises, and do not require acquisition of practice eyes and facilities to support biological specimens. Trainees may position themselves at the simulator as they would be sitting in the operating room. A realistic microscope and instrument foot pedals as well as instruments for each hand are incorporated into the simulator. For the novice surgeon, positioning at the microscope, managing instrumentation, and maneuvering in the operative field simultaneously can present a major challenge. The simulation of using all extremities at once can be a very valuable experience especially if aided with faculty feedback (see Figs. 31.7 and 31.8).
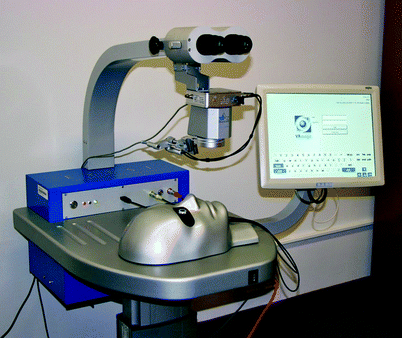




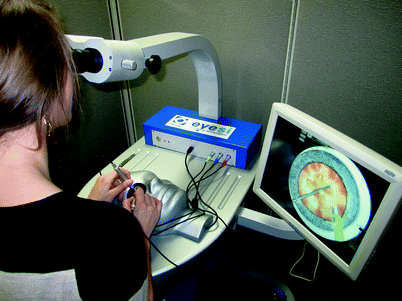

Fig. 31.3
The Eyesi® simulator. The system has oculars and operating scope model. The adjacent monitor will project the same view as the participant views through the ocular. There are two foot pedals designed in a similar fashion to the operating scope: foot pedal and the phacoemulsification machine pedal. The head can be positioned so that the operator can sit either temporally or superiorly

Fig. 31.4
The Eyesi® simulated patient and eye. Each of the ports on the outer aspect of the eye will allow for placement of one of the system’s instruments

Fig. 31.5
Examples of the “handpieces” of the Eyesi® simulator. Once placed through one of the ports of the model eye, the instrument will appear as the needed instrument for the task i.e., capsulorhexis forceps, irrigation cannula, and phacoemulsification hand piece

Fig. 31.6
The “handpieces” positioned within the simulated eye

Fig. 31.7
Example of a trainee positioned at the Eyesi®. You can see that the trainee is positioned as if she was in the operating room: each foot upon the appropriate foot pedal, chair and ocular height adjusted to allow comfort, and hands positioned with instruments in the eye. There is a projected view of what the trainee is seeing/doing on the computer monitor alongside the trainee permitting someone else to observe

Fig. 31.8
Example of a trainee positioned at the Eyesi®
Both computer simulation devices have a computerized eye model that includes a lens, iris, capsule, and cornea. The eye model is visible through the oculars, creating a stereo image to simulate the depth of the structures within the eye. The software senses the position of instruments and provides feedback or “grades” based on tissue handling and whether or not there was unintended damage of structures such as the cornea. The device provides an immediate numerical score on specific tasks (Capsulorhexis, Nucleofractis). Which can be used to track the progress of the trainee (see Figs. 31.9, 31.10, and 31.11).
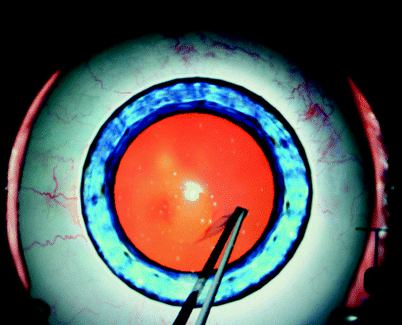
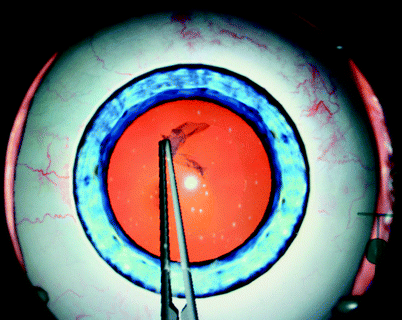
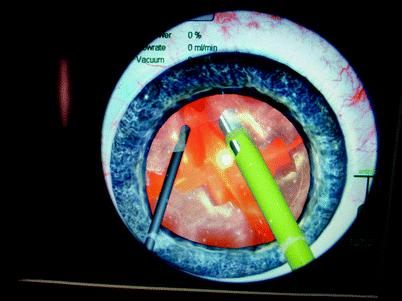

Fig. 31.9
Eyesi® simulation of creating a capsulorhexis

Fig. 31.10
Eyesi® simulation of creating a capsulorhexis

Fig. 31.11
Eyesi® simulation of nucleofractis
Advantages of Virtual Simulation
As outlined above, a wet lab offers a wide variety of opportunities for the trainee to practice surgical technique. However, setup for the wet lab often requires significant time and sometimes preparation of the tissue itself. This limits the ability to rapidly repeat a specific task, such as creating the capsulorhexis. With a wet lab, trainees may only be able to practice the capsulorhexis 2 or 3 times in an evening session before the biological eye is no longer usable to tissue disruption. With the Eyesi simulator, the resident can deliberately practice the capsulorhexis 100 times in an evening. The resident or faculty can set up technically difficult situations to allow for repeated practice which is not limited by setup time or delicate tissue disruption.
Another drawback to the wet lab is that for the training ophthalmic surgeon, feedback on performance is essential. A trainee can perform a given task repeatedly, but if they are executing the task improperly or stressing other tissues, then the practice of the improper technique is not beneficial. To ensure this does not occur, an experienced educator would need to be present observing and providing feedback to the trainee, which would require a significant amount of dedicated faculty time. While faculty presence is important in certain phases of computer simulation training, much can be done without faculty supervision after a set of exercises are established and real-time scoring is enabled. The simulators have software developed to “grade” the performance of trainees, and the trainee can indepedancy monitor their progress very easily and use this to train to competency.
The Evidence for Virtual Simulation
Several studies have demonstrated content and construct validity of the Eyesi®simulator. Mahr showed that the simulator’s tremor and forceps module had validity [28]. Using the forceps module, experienced surgeons achieved statistically significant better total scores, with lower total task time and instrument-in-eye measurements than did novices. For the anti-tremor module, experienced surgeons also achieved statistically significant better total scores, with lower task time and instrument-in-eye time measurements than did novices. As such, the Eyesi simulator represents a very useful device with established validity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








