and Richard A. Jaffe2
(1)
David Geffen School of Medicine at UCLA, Los Angeles, California, USA
(2)
Stanford University School of Medicine, Stanford, California, USA
Keywords
FluorideCompound ASolubilityInhalation inductionOrgan effectsComplicationsIntroduction
Over the past 50 years there have been three gas and 13 volatile anesthetic agents made available for clinical use.
Inhalation anesthetics used over last 50 years |
|---|
Nitrous oxide* |
Ethylene |
Cyclopropane |
Diethyl ether |
Chloroform |
Ethyl chloride |
Divinyl ether (Vinethene) |
Ethyl vinyl ether (Vinamar) |
Trichlorethylene (Trilene) |
Fluroxene |
Halothane (Fluothane)* |
Methoxyflurane (Penthrane) |
Enflurane |
Isoflurane (Forane)* |
Sevoflurane (Ultane)* |
Desflurane (Suprane)* |
Of the three gas anesthetics only nitrous oxide continues to be used widely, primarily because of its effectiveness, ease of administration, and low cost. Among the 13 other agents, based on both scientific data from the literature and extensive, personal clinical experience with all 13 that sevoflurane is the best volatile anesthetic ever developed. What follows will be a brief history of the drug, and consideration of those attributes that make is such a valuable anesthetic.
Historical Development
The development of sevoflurane is an unusual and truly remarkable story. In the late 1960s, four investigators at Travenol Laboratories in Morton Grove, Illinois began evaluating the inhalation anesthetic properties of a group of halogenated methyl isopropyl ethers. Their hope was to find an inhalation anesthetic that would be superior to halothane, which dominated the market at that time [1]. They investigated a number of compounds, the most promising being fluromethyl 2,2,2-trifluoro-1-[trifluoromethyl] ethyl (Fig. 11.1), which was given the name sevoflurane. Subsequent investigators Thomas Cook, Richard Mazze and Michael Halsey performed a variety of studies in mice, rats and dogs, administering sevoflurane for brief or prolonged periods and with single or multiple exposures without observing any serious sequellae [2]. However, two features about the drug emerged that were of concern. The first was that sevoflurane underwent biotransformation, which resulted in increases in inorganic fluoride ions in 24-h urine samples. The urinary inorganic fluoride levels were higher in mice given sevoflurane than those given halothane or isoflurane, but far below levels produced by methoxyflurane. The second concern was the fact that sevoflurane reacted with soda lime to form two substances identified by mass spectroscopy as compounds A and B.
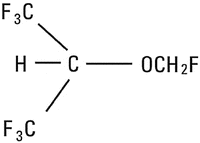

Fig. 11.1
Chemical structure of sevoflurane
Despite the favorable findings in the original report by Wallin et al. [1], there was very little interest in studying this anesthetic further, probably for three reasons. First, there was a longstanding, hallowed dictum in anesthesiology, which decried the development or use of any anesthetic that reacted with soda lime, regardless of what the derived product(s) might be. This tenet was based on prior experience with trichloroethylene, which decomposed to phosgene, hydrochloric acid, and carbon monoxide when exposed to warm soda lime for several hours. Clinical reports of cranial nerve palsies, particularly the fifth cranial nerve terminated its use soon after halothane became available. The second reason relates to the fact that Mazze and colleagues had just published their findings with methoxyflurane, which showed a dose-related nephrotoxicity in rats owing to its biotransformation to inorganic fluoride ions. While preliminary studies indicated that inorganic fluoride ion levels with sevoflurane were nowhere near as high or as prolonged as with methoxyflurane, neither halothane nor isoflurane, a newly developed volatile anesthetic at that time, caused any appreciable increase in serum or urinary fluoride levels. And third, since halothane remained a suitable drug for inhalation induction anesthesia, and isoflurane underwent less biotransformation than sevoflurane (1 vs. 3 %), and was a suitable maintenance agent, there was little incentive to investigate sevoflurane further.
However, Duncan Holaday and Burnell Brown were attracted to sevoflurane because of its pleasant smell, low blood-gas solubility (0.65), and hence rapid induction and recovery, and lack of cardiac arrhythmias when epinephrine is administered. In 1981 Holaday and Smith published a phase-1 study of the clinical characteristics and biotransformation of sevoflurane in six healthy volunteers exposed to 2–3 % for 1 h [3]. They observed stable respiratory and cardiovascular function, rapid induction and emergence with no aberrant behavior (coughing, shivering, retching, laryngospasm), limited biotransformation (about 3 % of the administered drug), and no toxic effects. Inorganic serum fluoride levels averaged 22 μmol/l, well below the values seen with methoxyflurane, and returned to normal values within 24 h after termination of the anesthetic. Their conclusion was that phase-2 and phase-3 clinical trials should commence. This didn’t happen. For the next 10 years no further clinical studies of sevoflurane were conducted. It was not until 1991 that Yasuda et al. published a study in which they compared the kinetics of sevoflurane and isoflurane in man [4]. In fact, Brown and Frink wrote an editorial in 1992 asking “Whatever happened to sevoflurane?” What happened was that the drug got lost in the shuffle as interest became directed toward the development of better intravenous anesthetics [5]. Baxter Travenol sold sevoflurane to the British Oxygen Corporation, which reorganized and changed its name to Ohmeda. Ohmeda, knowing that desflurane was in the final developmental stages sold sevoflurane to Mariushi, a Japanese pharmaceutical company. The drug was licensed in Japan and subsequently administered to several million Japanese without any apparent, serious complications. Based on this favorable experience, Mariushi Pharmaceuticals contracted with Abbott Laboratories to facilitate approval of the drug by the FDA, and subsequent distribution in the United States. Many phase 2 and 3 studies were done, and the drug was approved by the FDA in 1994, and became widely available in the United States in 1995.
Pharmacological Properties of Sevoflurane
Solubility
What are the special pharmacological characteristics of sevoflurane that make it the most valuable volatile anesthetic ever developed? There are several. Most important among these is its low blood-gas partition coefficient of 0.65.
Favorable characteristics of Sevoflurane |
|---|
Low blood-gas solubility coefficient (0.65) |
Rapid induction and recovery |
Potent, can be used with oxygen only |
Pleasant, non-irritating smell |
Minimal or no stimulation of airway reflexes |
Laryngospasm during induction or emergence uncommon |
Suitable for inhalation induction in all ages |
Standard, agent-specific vaporizer used |
Cardiovascular and respiratory functions maintained |
Protects heart from ischemia |
Compatible with epinephrine and norepinephrine |
This means that for every sevoflurane molecule in the blood phase, there are nearly two molecules in the gas phase. As a result both induction and emergence from sevoflurane anesthesia are rapid. Because sevoflurane has a slightly higher blood-gas partition coefficient than nitrous oxide (0.65 vs. 0.47), clinicians often discontinue sevoflurane near the conclusion of an anesthetic, but continue with nitrous oxide administration, expecting that with this technique the patient will emerge from anesthesia faster. While possibly true, this is a misguided practice for several reasons. First, the difference in emergence time between the two drugs is trivial (<5 min), and differences in recovery responses among patients are greater than the solubility difference between the two anesthetics. Second , it is much easier and safer to have a patient transition from controlled ventilation to spontaneous breathing with the lungs full of oxygen than with lungs containing nitrous oxide 50–60 %. Provided the anesthesiologist avoids airway obstruction via an endotracheal tube or LMA, substantial loss of lung volume from abdominal or thoracic compression, or inadequate reversal of neuromuscular blockade, a patient can go for a prolonged period without breathing and not sustain any degree of oxygen desaturation when the lungs are filled with oxygen-sevoflurane. The same is not true when the lungs contain nitrous oxide 50–60 %. When the lungs contain a substantial concentration of nitrous oxide, the anesthesia provider must watch the pulse oximeter closely, and ventilate the lungs regularly to avoid oxygen desaturation. This requirement for periodic ventilation delays return of spontaneous ventilation because it slows the rate of carbon dioxide accumulation and hence the stimulus to breathe. Thirdly, if patients should inadvertently cough or buck on an endotracheal tube or LMA during the transition from controlled to spontaneous ventilation, the severity of oxygen desaturation is worse when the lungs contain nitrous oxide than when they contain sevoflurane-oxygen. And lastly, if a patient should become unacceptably light during the terminal phases of an operation, the anesthesia provider can increase the sevoflurane concentration and total oxygen flow, and the level of anesthesia will very rapidly deepen to an acceptable level. The reasons that this works so quickly are twofold. First, the patient already has substantial venous and tissue concentrations of sevoflurane, so very little must to be added to the alveolar, arterial blood and brain concentrations to achieve a deeper plane of anesthesia. And second, the low solubility coefficient makes the uptake of sevoflurane rapid. The same is not true when nitrous oxide is used. Given its lower potency, one cannot increase the nitrous oxide concentration safely to a level that will resolve the need for more anesthesia, so an intravenous drug (e.g. propofol, thiopental, lidocaine) must be administered. Depending upon the dose administered, and other prevailing factors, the patient may become apneic necessitating controlled ventilation for a time.
Patient safety is greatly enhanced by discontinuing nitrous oxide rather than sevoflurane near the conclusion of a nitrous oxide-sevoflurane anesthetic.
In contrast to human tissues, sevoflurane is soluble in soda lime, and five times more soluble in baralyme, with the solubility increasing in both absorbents as absorbent temperature increases. However, it has been shown that the absorption of sevoflurane in soda lime has minimal effects on the clinical characteristics of the drug.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






