Increase in serum creatinine level (mg/dL)
Multivariable OR (95 % CI)
Area under ROC curve
Increase in total cost
0.3
4.1(3.1–5.5)
0.84
$4886
0.5
6.5(5.0–8.5)
0.86
$7499
1.0
9.7(7.1–13.2)
0.84
$13,200
2.0
16.4(10.3–26)
0.83
$22,023
Definitions
Historically, acute decline in renal function was known as acute renal failure. This term has been replaced with “acute kidney injury “as a simple and direct description of the process, one that patients can readily understand and can be interpreted as a spectrum of disease, including mild or moderate dysfunction along with outright failure. The term injury is thought to better represent the pathologic changes occurring in the kidney parenchyma [12]. Unfortunately, AKI has over 35 definitions, many of which are complex and confusing. This lack of standardization has greatly impeded the understanding of the epidemiology of kidney dysfunction. Many attempts have been made to provide clinicians a standardized definition; unfortunately, with complexity of the disease, there are pitfalls to each definition [12].
One commonly accepted definition is the RIFLE criteria. It was developed by a multidisciplinary group to address the issue of a uniform definition of AKI. The acronym RIFLE represents three severity levels of renal dysfunction (Risk, Injury, and Failure) and two outcome classifications (Loss of kidney function and End-stage kidney disease) based on measurements of creatinine and urine output (UO). The impairment must be sudden and sustained (>24 h). The dysfunction criteria are derived from the relative increase in serum creatinine, the absolute level of UO, or both. The failure category uses serum creatinine (SCr) ≥4 mg/dL to account for the severity of acute renal disease in patients with chronic renal disease whose relative increase in creatinine may not otherwise reflect AKI (Table 37.2). Unfortunately, the RIFLE criteria are most useful when the baseline SCr is known, but, in trauma patients, this may not be available [13].
Table 37.2
Acute kidney injury criteria: RIFLE and KDIGO
RIFLE | Laboratory criteria | UOP criteria | KDIGO stage | Laboratory criteria | UOP criteria | |
|---|---|---|---|---|---|---|
 | Risk | Cr 1.5× baseline or GFR decrease >25 % | <0.5 mL/kg/h ×6 h | 1 | 1.5–1.9× baseline or >0.3 mg/dL increase | <0.5 mL/kg/h ×6–12 h |
Injury | Cr 2× baseline or GFR decrease >50 % | <0.5 mL/kg/h ×12 h | 2 | 2.0–2.9× baseline | <0.5 mL/kg/h for >12 h | |
Failure | Cr 3× baseline, Cr >4 mg/dL or GFR decrease >75 % | <0.5 mL/kg/h ×12 h or anuria ×12 h | 3 | 3.0× baseline or serum Cr >4.0 mg/dL or initiation of RRT | <0.3 mL/kg/h for >24 h or anuria for >12 h | |
Loss | Complete loss of renal function for >4 weeks | |||||
End-stage renal disease | Complete loss of renal function for >3 months |
To address some of the shortcomings of the RIFLE criteria, the Kidney Disease: Improving Global Outcomes (KDIGO) examined several definitions of AKI and developed an easy, clinically applicable definition for AKI. Criteria include an increase in SCr >0.3 mg/dL within 18 h or an increase in SCr to > 1.5 times baseline, which is known or presumed to have occurred within the past 7 days or UO of <0.5 mL/kg/h for 6 h.
To grade the severity of AKI, three levels of dysfunction were identified. Stage 1 includes a SCr increase of 1.5–1.9 times baseline or ≥0.3 mg/dL or UO <0.5 mL/kg/h for 6–12 h. Stage 2 is defined by any of the following, an increase in SCr of 2.0–2.9 times baseline, an increase of SCr ≥ 4 mg/dL, or UO <0.5 mg/kg/h for ≥ 12 h. Stage 3 includes any of the following, an increase of SCr over 3.0 times baseline, initiation of RRT, estimated glomerular filtration rate (eGFR) decrease to <35 mL/min per 1.73m2 in patients less than 18 years, or anuria for ≥ 12 h (Table 37.2) [14].
Risk Factors
The etiology of AKI is often multifactorial in geriatric patients. Age-related changes of the kidney make the elderly more susceptible to AKI at baseline [15]. In the trauma setting, hypovolemia from pre-existing medical problems, under-resuscitation, and shock from hemorrhage play important roles in increasing the risk of AKI. Comorbidities such as diabetes, hypertension, cardiovascular disease, congestive heart failure, and especially baseline chronic kidney disease (CKD) are additional independent risk factors for developing, and not recovering from, AKI. Furthermore, many patients with comorbid medical conditions are treated with agents that may be nephrotoxic and predispose the elderly trauma patient to AKI (Fig. 37.1) [16].
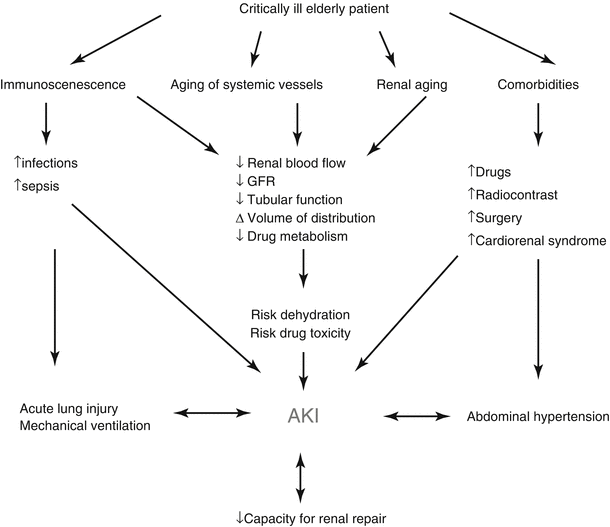

Fig. 37.1
Factors contributing to AKI (Adapted from Chronopoulos et al. [16]. Reproduced, with permission from Springer Science + Business Media)
Age-Related Changes of the Kidney and Risk of AKI in the Elderly
As patients age, there are structural alterations to the kidney. These include atherosclerotic and mesangial matrix changes which alter blood flow. Starting at the age of 40 years, renal blood flow can decrease by approximately 10 % per decade. The vascular tone of the kidney is maintained by renin-angiotensin-aldosterone system and vasodilatory prostaglandins. There is a reduction of these vasodilatory prostaglandins that can lead to changes in renal vascular tone and exaggerated response to vasoconstrictive stimuli. This can lead to increased vulnerability to stressors such as hypovolemia, low-flow states, or medications that affect the hormonal regulation of the kidney [1, 2].
Structural changes inside the functional renal parenchyma occur as a part of the normal senescence process. Fibrous tissue replaces normal glomerular tissue over time, resulting in a 30–50 % loss of cortical glomeruli by the age of 70 years. Tubular atrophy, reduced tubular number and length, fatty degeneration, and fibrosis lead to fragility of the tubule making them less tolerant to injury caused by toxins or hypoxia. Aging and cellular senescence lead to a loss of 4500 nephrons per kidney per year. The result is a decreased renal mass; by the age of 80 years, 25–30 % of mass is lost [2].
Due to the process of aging, renal function declines, often starting after the third decade of life. The normal loss of glomerular filtration rate (GFR) is estimated at 0.8–1.0 mL/min/1.73 m2 per year. With age comes loss of skeletal muscle or sarcopenia estimated at 0.5–1% per year starting at the age of 25 years. The process of sarcopenia results in a decrease production of creatinine. The progressive decrease in GFR is not necessarily reflected in a concomitant increase in serum creatinine due to the loss of creatinine production; thus, creatinine is not an ideal marker for renal functional decline [2].
An important function of the kidney is maintaining electrolyte and water homeostasis. As the kidney ages, there is a reduction in its ability to regulate these important processes through changes in the loop of Henle and other structures. Sodium regulation is greatly affected due to a blunted response to large sodium loads and a decrease ability to compensate for sodium loss. Approximately 85 % of elderly persons have decreased ability to reabsorb sodium. The normal countercurrent exchange in the kidney is gradually lost, resulting in renal medullar hypotonicity. The hypotonic state causes reduced ability to maximally concentrate urine which can lead to dehydration. This leads to decreased responsiveness to antidiuretic hormone (ADH) leading to a reduction of water absorption. Senile sodium leakage and water disequilibrium due to a reduction in free water excretion can lead to chronic hyponatremia. Water equilibrium is altered through a decreased response of ADH release and thirst regulation. Due to declining number of urea channels in the tubules, reduction of urea reabsorption occurs, which can lead to a loss of water through osmotic diuresis. Additionally, decreased aldosterone production and developing resistance to aldosterone in the tubule impair potassium regulation. Thus, elderly patients become more susceptible to the development of hyperkalemia [1, 2].
Chronic Kidney Disease and Risk of AKI in the Elderly
CKD is described by a sustained eGFR less than 60 mL/min/1.73 m2 [2]. Patients with AKI, superimposed on CKD, have a 41-fold increase risk to progress to end-stage renal disease (ESRD) compared to a 13-fold increase in patients with AKI without CKD. Age older than 65 is a risk factor for failure to recover from AKI and even progression to advanced-stage CKD [17, 18]. Critically ill surgical patients that recover from an initial episode of AKI are at a higher risk for subsequent AKI, which carries higher mortality and a decline in renal function at the time of subsequent discharge [19].
Volume Status and Risk of AKI in the Elderly
Intravascular hypovolemia leads to renal under-perfusion from decreased effective arterial blood volume and can subsequently cause AKI. Prerenal etiologies account for approximately one-third of AKI in the elderly [1]. Baseline intravascular hypovolemia from congestive heart disease or liver disease increases the risk of ischemic and nephrotoxic insults in elderly patients who already have physiologic age-related changes as outlined above [15]. Traumatic hemorrhage, third spacing of fluids after operative interventions, and sepsis may result in intravascular volume depletion and thus also increase the risk of AKI. Medications such as diuretics, NSAID, and angiotensin-altering medications and changes in nephron sodium handling may alter the kidneys’ autoregulatory responses in the setting of volume loss [1]. For example, Kim et al. showed that among 153 adults who received treatment for intracranial hypertension from TBI, high doses of mannitol (≥1.34 g/kg/day) were associated with more frequent and severe AKI. This was particularly evident in patients over 69 years and pre-existing renal dysfunction [20].
When treating hypovolemia, the goal should be euvolemia, as overly aggressive fluid resuscitation can be harmful [21]. The neurohormonal effects of trauma including catecholamine release and sympathetic activity stimulation result in sodium and water retention which persists after the inciting stressor resolves, leading to excessive volume accumulation [22]. Fluid overload results in tissue edema. This impairs oxygen and metabolite diffusion, distorts tissue architecture, obstructs capillary blood flow and lymphatic drainage, and disturbs cell-cell interactions that may then contribute to progressive organ dysfunction, including AKI. Cardiogenic edema can worsen forward flow, altering renal blood flow and risking injury to the kidney. Additionally, hypoxia induced by pulmonary edema can result in inadequate kidney oxygenation causing further injury [22, 23].
Hypervolemia associated with resuscitation can predispose a patient to intra-abdominal hypertension (IAH). It is defined as repeated or sustained intra-abdominal pressure (IAP) ≥12 mmHg. This is thought to be due to large volumes during resuscitation, and capillary leak leads to visceral edema and IAH. The increase pressure decreases renal perfusion pressure, inducing a “renal hypovolemic state,” and has been shown to decrease renal artery resistive index and UO. An IAP of 20 mmHg can reduce the renal blood flow by 20–25 %. Another pressure phenomenon that affects renal perfusion through the same “renal hypovolemic state” is positive pressure ventilation (PPV). PPV can reduce cardiac output by decreasing preload and activate renin-angiotensin axis. One study revealed that invasive mechanical ventilation was associated with a threefold increase in the odds of developing AKI in critically ill patients [24].
Infection
Elderly are at increased risk for healthcare-associated infections, and they also have an increased risk of developing AKI from sepsis [16, 25]. The BEST Kidney investigators reported in their prospective observational study of over 29,000 ICU patients that nearly half of the 5.7 % who developed AKI had septic shock as a likely etiology [26, 27]. Septic AKI is emerging as a separate entity with distinct pathophysiology and outcomes [28]. White et al. examined 246 septic surgical patients and found AKI (defined by RIFLE criteria) occurred in 67 % of the patients, with higher rates among those with septic shock (88 %). Patients with AKI had statistically significant longer ventilator and ICU days and increased hospital mortality and were less likely to be discharged home [29].
Nephrotoxins and the Risk of AKI in the Elderly
Drug-induced nephrotoxicity is another major cause of AKI in the elderly [15, 17]. Diuretics and angiotensin-altering drugs used to treat hypertension and congestive heart failure deplete volume which can cause AKI. NSAIDs are associated with acute tubular necrosis or acute interstitial nephritis. New NSAIDs used in patients over 65 years double the risk for AKI. Additionally, they have a longer half-life in the elderly, further increasing their risk of acute kidney injury [17]. Hypersensitivity to medications causes acute interstitial nephritis, a less common cause of AKI. Antibiotics, especially penicillins, cephalosporins, and sulfonamides, are often implicated [1, 30].
Contrast-induced nephropathy (CIN) has been implicated as a major cause of AKI in hospitalized patients [1, 31]. Several factors increase this risk including hypotension, sepsis, coadministration of other nephrotoxic agents, diabetes, pre-existing renal dysfunction, and higher injury severity score in trauma patients [1, 32–34]. A retrospective study of 1371 trauma patients done by McGillicuddy et al. showed that receiving IV contrast did not increase the rate of AKI [35]. Many of the previous risk factors for AKI were not accounted for in this study. The body of evidence does suggest contrast agents can contribute to the development of AKI. It is likely that AKI in the setting of IV contrast administration is multifactorial, given many different patient factors that can predispose a patient to develop AKI rather than the contrast agent alone [36–38]. It is important to realize the potential for CIN among elderly trauma patients; using these agents judiciously is responsible medicine.
Trauma patients are at risk for rhabdomyolysis. Myoglobin release from ischemic muscle induces renal vasoconstriction, formation of intratubular casts, and the direct toxicity of myoglobin to the kidney tubule [39].
Anatomic Causes of AKI
Postrenal obstruction occurring proximal to the level of the ureter, the bladder, or at the bladder outlet/urethra causes up to 10 % of AKI. Postrenal obstruction AKI typically occurs in men from benign prostatic hypertrophy or prostate cancer. In women, retroperitoneal and pelvic malignancies are the most important causes. Neurogenic bladder and medications such as anticholinergics can lead to obstructive uropathy. Obstruction related to trauma can be due to a direct injury to the genitourinary tract (i.e., bladder hemorrhage with clot); space-occupying retroperitoneal lesions such as hematomas or abscesses can also cause ureteral or bladder obstruction in trauma patients [1]. The kidney can be directly injured by the traumatic insult. In trauma to the kidney, renal dysfunction has been directly correlated to the severity injury [40].
Preventing AKI
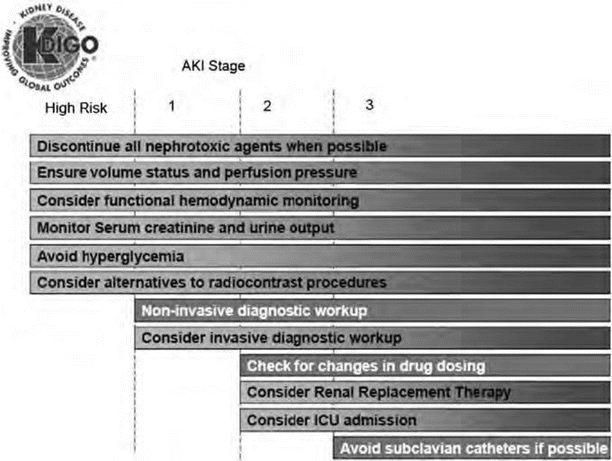
In 2012, KDIGO issued guidelines to prevent AKI in adults based on a systematic review of renal-protective strategies in the literature (Table 37.3). Some of their key recommendations include (level of evidence):
Volume expansion: KDIGO recommends controlled fluid resuscitation with isotonic crystalloids in volume depletion (grade 2B) in the absence of hemorrhagic shock. They recommend against the use of diuretics to prevent or ameliorate AKI (grade 1B evidence). Patients at high risk of CIN should receive intravenous volume expansion with either isotonic sodium chloride or sodium bicarbonate solutions, rather than oral fluids alone (1C), or no intravenous volume expansion (1A).
Vasopressors: The group recommends the use of vasopressors in conjunction with fluids in patients with vasomotor shock with or at risk for AKI (1C). They suggest against using low-dose dopamine to protect against or treat AKI (grade 1A).
Vasodilators: The group recommends against the use of fenoldopam to prevent or treat AKI or CIN (2C, 1B).
Metabolic/Endocrine: They recommend insulin therapy to obtain a plasma glucose of 110–149 mg/dL in the critically ill (2C). Protein restriction should be avoided with the aim being preventing or delaying RRT (2D). They suggest adequate nutritional support for all patients at risk for AKI via an enteral route (grade 2C). The use of n-acetylcysteine (NAC) should be avoided to prevent AKI in patients with hypotension (2D) or after surgery (2D). Oral NAC can be considered to prevent CIN in high-risk patients in conjunction with isotonic intravenous fluids (2D).
Volume status management/hemodynamic support |
Controlled fluid resuscitation with isotonic crystalloids in volume depletion (2B) |
Use of blood products in hemorrhagic shock |
Avoid the use of diuretics to prevent or ameliorate AKI (1B) |
Except volume overload states (2C) |
Use of vasopressors in conjunction with fluids in patients with vasomotor shock with or at risk for AKI (1C) |
Avoid using low-dose dopamine to protect against or treat AKI (1A) |
Avoid the use of fenoldopam to prevent or treat AKI or CIN (2C, 1B) |
Metabolic/endocrine support |
Insulin therapy may be needed to obtain plasma glucose of 110–149 mg/dL in the critically ill (2C) |
Adequate nutritional support for all patients at risk for AKI via an enteral route when possible (grade 2C) |
Total energy intake of 20–30 kcal/kg/d in patients |
Protein restriction should be avoided with the aim being preventing or delaying RRT (2D) |
Protein calculations of 0.8–1.0 g/kg/d in non-catabolic AKI patients without the need for RRT (2D) |
Protein calculations of 1.0–1.5 g/kg/d in patients with AKI on RRT (2D) |
Maximum of 1.7 g/kg/d in patients on CRRT or hypercatabolic patients (2D) |
Prevention of CI-AKI |
Consider alternative imaging studies in patients at increased risk |
Use the lowest possible dose of contrast medium in patients at increased risk (NG) |
Iso-osmolar or low-osmolar iodinated contrast media is preferred over high-osmolar (1B) |
Intravenous volume expansion with either isotonic sodium chloride or sodium bicarbonate solutions rather no intravenous fluids in patients at increased risk (1C) |
Not using oral fluids alone in patients at increased risk (1A) |
N-acetylcysteine (NAC) should be avoided to prevent AKI in patients with hypotension (2D) or postsurgical (2D). Oral NAC can be considered in high-risk patients in conjunction with isotonic intravenous fluids (2D) |
Evaluation of AKI
A detailed history with particular attention to diabetes, hypertension and vasculitides, baseline renal function, hepatic dysfunction, and potential nephrotoxic medications is essential in assessing the risk for AKI in any given patient. Physical examination should focus on the assessment of volume status and signs of uremia. Diagnostic studies should include standard serum complete blood counts, chemistry panels, myoglobin, urine electrolytes, and urine sediment examination. Renal ultrasound is useful to rule out obstructive causes. UO should be monitored closely and urinary catheter may be necessary. In rare instances, kidney biopsy is necessary to truly establish the diagnosis [15, 41].
Treatment
Treatment for AKI is supportive. Figure 37.2 provides stage-based management recommendations per KDIGO. A critical step in treating AKI is to avoid exposure to nephrotoxins, maintain appropriate hemodynamic parameters, avoid hypoxia, and minimize repeated episodes of AKI, which increase the risk of non-recovery of renal function, especially in the elderly. One of the first steps is to ensure euvolemia in AKI through the use of fluid resuscitation or blood product administration in hemorrhage shock. Isotonic crystalloid fluids over colloid are preferred. Vasopressors may be needed to maintain goal hemodynamics in conjunction with fluids in shock states. Avoiding hyperglycemia is important to prevent osmotic diuresis and infectious complications. Adequate nutrition needs to be ensured, preferably via the enteral route with particular attention to the need for increased protein in patients on RRT or in hypercatabolic states. Table 37.3 provides information from KDIGO for management of AKI [14].
Diuretics in ARF
The physiologic argument to use diuretics in critically ill patients with AKI is that diuretics increase urinary flow and renal perfusion which decreases the risk of ongoing injury. Loop diuretics are also thought to decrease metabolic demand of renal tubular cells and reduce oxygen consumption. Furthermore, some suggest that oliguric AKI is associated with higher mortality than nonoliguric AKI [42]. Karajala et al. conducted a systematic review in 2009 evaluating the role of diuretics in treating AKI and found that diuretics, while useful in managing volume status, do not improve mortality, decrease the use of RRT, or speed recovery from AKI [43]. Diuretics have a role in the setting of oliguric AKI, if systemic complications of volume overload such as pulmonary edema are manifest [43, 44]. According to KDIGO, the use of diuretics should not be aimed at treatment of AKI itself but rather volume overload associated with the disease [14].
Indication for RRT
There are two potential indications for RRT in critically ill patients: renal replacement and renal/multi-organ support. RRT can correct electrolyte imbalances and acid/base disturbances and improve volume status and uremic complications [45]. Examples of conditions that may improve with renal support include congestive heart failure, respiratory acidosis from adult respiratory distress syndrome, liver failure, pancreatitis, fluid management in multi-organ failure, lactic acidosis, crush injury, tumor lysis syndrome, and, potentially, cytokine removal in sepsis [46, 47].
Initiation of RRT in an oliguric patient generally requires evidence of severe hyperkalemia, severe pulmonary edema, severe academia, uremic complications, and intoxication of dialyzable substances, although specific guidelines for thresholds are lacking [48]. Continuous renal replacement therapy (CRRT), rather than intermittent hemodialysis (IHD), is often preferred for critically ill patients with hemodynamic instability [24].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree