Chapter 41 Renal replacement therapy
When acute renal failure (ARF) is severe, resolution can take several days or weeks. During this time, the kidneys cannot maintain homeostasis of fluid, potassium, metabolic acid and waste products. Life-threatening complications frequently develop. In these patients, extracorporeal techniques of blood purification must be applied to prevent such complications. Such techniques, broadly named renal replacement therapy (RRT), include continuous haemofiltration (HF), intermittent haemodialysis (IHD) and peritoneal dialysis (PD), each with its technical variations. All of these techniques rely on the principle of removing unwanted solutes and water through a semipermeable membrane. Such membrane is either biological (peritoneum) or artificial (haemodialysis or HF membranes), and each offers several advantages, disadvantages and limitations.
PRINCIPLES
The principles of RRT have been extensively studied and described.1–3 This is a summary of some aspects which are relevant to the critical care physician.
WATER REMOVAL
SOLUTE REMOVAL
The removal of unwanted solute can be achieved by:
INDICATIONS FOR RENAL REPLACEMENT THERAPY
In the critically ill patient, RRT should be initiated early, prior to the development of complications. Fear of early dialysis stems from the adverse effects of conventional IHD with cuprophane membranes, especially haemodynamic instability, and from the risks and limitations of continuous or intermittent PD.4–5 However, continuous RRT (CRRT)6,7 or slow low-efficiency daily dialysis (SLEDD)8 minimises these effects. The criteria for the initiation of RRT in patients with chronic renal failure may be inappropriate in the critically ill.9,10 A set of modern criteria for the initiation of RRT in the intensive care unit (ICU) is presented in Table 41.1.
Table 41.1 Modern criteria for the initiation of renal replacement therapy (RRT) in the intensive care unit*
| Oliguria (urine output < 200 ml/12 h) |
| Anuria (urine output: 0–50 ml/12 h) |
| [Urea] > 35 mmol/l |
| [Creatinine] > 400 μmol/l |
| [K+] > 6.5 mmol/l or rapidly rising† |
| Pulmonary oedema unresponsive to diuretics |
| Uncompensated metabolic acidosis (pH < 7.1) |
| [Na+] < 110 and > 160 mmol/l |
| Temperature > 40oC |
| Uraemic complications (encephalopathy/myopathy/neuropathy/pericarditis) |
| Overdose with a dialysable toxin (e.g. lithium) |
* If one criterion is present, RRT should be considered. If two criteria are simultaneously present, RRT is strongly recommended.
† Please be aware of differences between plasma versus serum measurement in your laboratory.
With IHD, CRRT or SLEDD there are limited data on what is ‘adequate’ intensity of dialysis. However, this concept should include maintenance of homeostasis at all levels,10 and better uraemic control may translate into better survival.11,12 An appropriate target urea might be 15–25 mmol/l, with a protein intake around 1.5 g/kg per day. This can be easily achieved using CRRT at urea clearances of 35–45 l/day depending on patient size and catabolic rate. If intermittent therapy is used, daily and extended treatment as described with SLEDD becomes desirable.13
MODE OF RENAL REPLACEMENT THERAPY
There is a great deal of controversy as to which mode of RRT is ‘best’ in the ICU, due to the lack of randomised controlled trials comparing different techniques. In their absence, techniques of RRT may be judged on the basis of the following criteria:
CRRT and SLEDD offer many advantages over PD and conventional IHD (3–4 h/day, 3–4 times/week),13 and whereas CRRT is almost exclusively used in some centres,14 only a minority of American ICU patients receive CRRT. Some salient aspects of CRRT, IHD and PD require discussion.
CONTINUOUS RENAL REPLACEMENT THERAPY
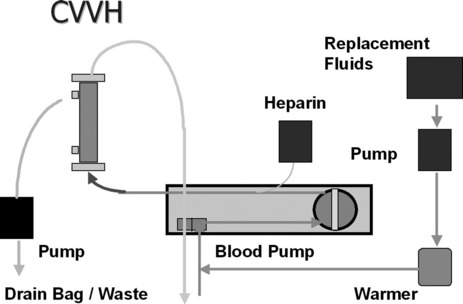
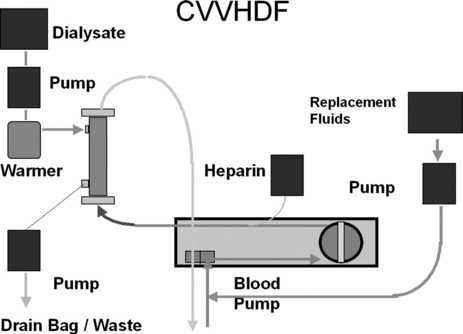
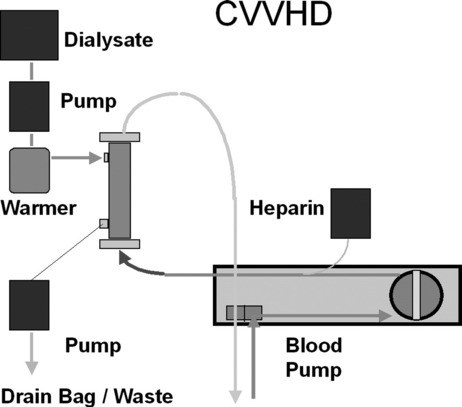
First described in 1977, CRRT has undergone several technical modifications. Initially, it was performed as an arteriovenous therapy (continuous arteriovenous HF: CAVH). The need to cannulate an artery, however, is associated with 15–20% morbidity. Accordingly, double-lumen catheters and peristaltic blood pumps have come into use (continuous venovenous HF: CVVH) with or without control of ultrafiltration rate. Ultrafiltration rates of 2 l/h yield urea clearances > 30 ml/min. Diagrams illustrating typical HF circuits are presented in Figures 41.1–41.3.
No matter what technique is used, the following outcomes are predictable:
DOSE OF CRRT AND CIRCUIT ANTICOAGULATION
The optimal dose (expressed as effective effluent per kilogram per hour) of CRRT remains unknown. Several studies suggest that higher dose may translate into better outcome.12,15 However such studies have been single-centre in nature and require confirmation in multicentre randomised controlled trials. Such trials are now under way (see below).16 It seems wise to wait for the results of larger studies before making any changes to the typical dose of approximately 25 ml/kg per hour currently delivered in Australia and New Zealand.
The flow of blood through an extracorporeal circuit causes activation of the coagulation cascade and promotes clotting of the filter and circuit itself. In order to delay such clotting and achieve acceptable operational lives (approximately 24 hours) for the circuit, anticoagulants are frequently used.17 However, circuit anticoagulation increases risk of bleeding. Therefore, the risks and benefits of more or less intense anticoagulation and alternative strategies (Table 41.2) must be considered.
Table 41.2 Strategies for circuit anticoagulation during continuous renal replacement therapy
| No anticoagulation |
| Low-dose prefilter heparin (< 500 IU/h) |
| Medium-dose prefilter heparin (500–1000 IU/h) |
| Full heparinisation |
| Regional anticoagulation (prefilter heparin and postfilter protamine, usually at a 100 IU:1 mg ratio) |
| Regional citrate anticoagulation (prefilter citrate and postfilter calcium – special calcium-free dialysate needed) |
| Low-molecular-weight heparin |
| Prostacyclin |
| Heparinoids |
| Serine proteinase inhibitors (nafamostat mesylate) |
In the vast majority of patients, low-dose heparin (< 500 IU/h) is sufficient to achieve adequate filter life, is easy and cheap to administer and has almost no effect on the patient’s coagulation tests. In some patients, a higher dose is necessary. In others (pulmonary embolism, myocardial ischaemia), full heparinisation may actually be concomitantly indicated. Regional citrate anticoagulation is very effective but requires a special dialysate or replacement fluid.18 Regional heparin/protamine anticoagulation is also somewhat complex but may be useful if frequent filter clotting occurs and further anticoagulation of the patient is considered dangerous. Low-molecular-weight heparin is also easy to give but more expensive. Its dose must be adjusted for the loss of renal function. Heparinoids and prostacyclin may be useful if the patient has developed heparin-induced thrombocytopoenia and thrombosis. Finally, in perhaps 10–20% of patients anticoagulation is best avoided because of endogenous coagulopathy or recent surgery. In such patients adequate filter life can be achieved provided that blood flow is kept at about 200 ml/min and vascular access is reliable.19
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree