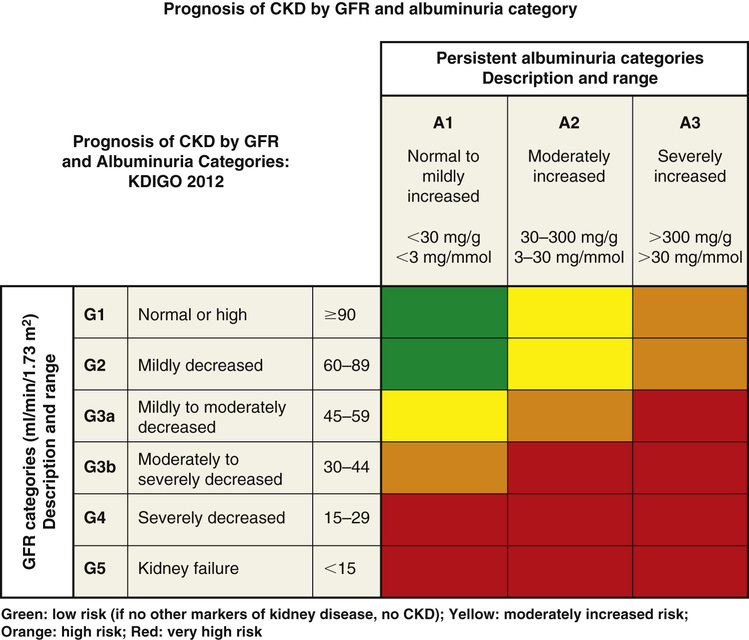
Chris Winkelman, Evelyn Duffy Kidney damage causes loss of filtration and the retention of waste products in blood. Patients with kidney damage experience a complex and common health condition that demands the involvement of both the primary care provider and specialists. The loss of kidney filtration has two patterns of damage: chronic kidney disease (CKD) and acute kidney injury (AKI). These patterns of damage may result in impaired kidney function, although AKI is considered potentially reversible. However, both AKI and CKD can also progress to kidney failure or the absence of kidney function. Kidney failure is also known as end-stage renal disease (ESRD) and stage 5 CKD. CKD, AKI, and kidney failure or ESRD replace other terms that have been used, such as renal insufficiency, acute renal failure, and acute-on-chronic kidney disease.1,2 In 2002, the National Kidney Foundation (NKF) published a landmark clinical practice guideline for the evaluation, classification, and stratification of CKD.3 This guideline provided a common language for classifying CKD based on glomerular filtration rate (GFR). This 2002 guideline also resulted in changes in how creatinine was measured and reported by laboratories, generated new codes for the International Statistical Classification of Diseases and Related Health Problems (ICD), improved estimation of GFR, and increased awareness on the part of providers of the importance of early identification of kidney damage. Over time, it became evident that the use of the guidelines needed refinement because some patients without kidney damage, particularly older adults, were diagnosed as having CKD based on GFR estimation. In 2012 an updated guideline was published.1 The Kidney Disease: Improving Global Outcomes (KDIGO) group refined the definition of CKD as “abnormalities in kidney structure or function for greater than 3 months with implications for health,” and this definition now includes measures of kidney damage detailed in Table 149-1.4 The 2012 guideline recommended that CKD be categorized based on the cause, GFR, and amount of albuminuria; these categories are illustrated in Tables 149-2 and 149-3.4 This international guideline was reviewed for its usefulness in the United States and was accepted by the NKF.4 TABLE 149-1 Guidelines: Diagnosing CKD TABLE 149-2 Categories of CKD TABLE 149-3 Albuminuria Categories in CKD The Centers for Disease Control and Prevention estimates that over 20 million Americans, 10% of the population, have CKD and that the majority of those are undiagnosed.5 Both providers and patients have demonstrated a lack of awareness of the diagnosis of kidney disease. Studies of provider and patient awareness of CKD have consistently found that the majority of cases are not recognized by providers and that patients themselves are unaware of their damaged kidneys.6,7 Although CKD is more prevalent in adults aged 65 years and older, about one third of older adults do not experience progressive disease.7,8 Similar to CKD, AKI has undergone a succession of definitions and refinement of stages. The most recent international source is from the KDIGO Acute Kidney Injury Work Group (2012).2 AKI is defined as an increase in serum creatinine of 0.3 mg/dL over 48 hours or an increase of serum creatinine to 1.5 times baseline over the prior seven days; or a urine volume output of less than 0.5 mL/kg/hr for 6 hours. According to this guideline, AKI severity is classed from 1 to 3, with 1 being the most severe as illustrated in Table 149-4. TABLE 149-4 Staging of AKI AKI is an abrupt loss of kidney function that occurs over 48 hours, although progressive damage may occur over as long as 7 days.2 AKI can progress to CKD when kidney damage persists for 3 months or longer. AKI occurs in as many as 30% of adults admitted to the intensive care unit and about 7% of all hospital admissions in the United States.9 AKI is a significant contributor to mortality and morbidity during hospitalization and in the year after discharge from acute care.10 Older adults, particularly those older than 75 years, are at increased risk for AKI and its complications.11 Both AKI and CKD can progress to kidney failure in a small but significant percentage of people.12 Kidney failure (ESRD) is characterized by anuria and the need for renal replacement therapy or kidney transplant. The kidneys and the urinary tract system no longer filter blood, create filtrate, or excrete urine in amounts sufficient to clear waste and balance fluid intake with output. In addition to being a clinical diagnosis, ESRD has administrative implications because those patients who receive dialysis or a kidney transplant as a result of this diagnosis are eligible for health insurance through Medicare regardless of their age. The basic pathophysiologic process of kidney disease is loss of functioning nephrons. As filtration capacity falls to less than 50%, the remaining healthy nephrons exhibit hyperfiltration and hypertrophy. This process is hypothesized to contribute to increased glomerular capillary pressure, leading to secondary nephron damage and progressive disease.13 The results are a decrease in GFR; an increase in circulating biomarkers of kidney disease, including creatinine and blood urea nitrogen (BUN); and albuminuria. The pathophysiology of AKI is any process that interferes with perfusion, filtration, or excretion.14 Disruption to perfusion is categorized as prerenal. Filtration abnormalities are intrinsic causes of AKI and refer to the pathology of vessels, glomeruli, or tubules and interstitium.15 Postrenal causes of AKI include obstruction to the renal pelvis, ureters, bladder, or urethra. However, AKI can also result from combined pathologic processes. For example, poor perfusion and subsequent ischemic damage to the nephron comprise a combined prerenal and intrinsic cause of AKI. Consider the hospitalized patient with prolonged poor renal perfusion (i.e., prerenal) from a hypotensive shock state (e.g., cardiogenic, septic, or hemorrhagic shock) with concurrent acute tubular necrosis (related to endotoxin exposure or ischemic necrosis of the nephron tubules), an intrinsic cause of AKI. As a second example of combined pathology, consider the patient with untreated kidney stones leading to urinary tract obstruction—postrenal AKI—leading to fibrosis and atrophy of the obstructed kidneys—an intrinsic pathology. Risk factors of comorbidities, age, and drugs can also contribute to prerenal, intrinsic, and postrenal AKI. For example, comorbidities that affect blood flow to the kidneys such as chronic heart failure (HF) or cirrhosis increase the risk for AKI. Hypovolemic states from acute blood loss, diarrhea, or dehydration lead to prerenal injury. Contrast from imaging studies may contribute to AKI.16 Small vessel vascular pathology from diabetes, clotting disorders, and hypertension contributes intrinsic structural changes leading to AKI.17 Renal artery stenosis from atherosclerosis or dysplasia can cause ischemic intrinsic nephropathy.16 Inflammatory conditions, sepsis, kidney infection, drug-induced nephritis, and malignancies damage tubules and the interstitium, leading to intrinsic causes of AKI.18–20 Postrenal (obstructive) AKI may be caused by neoplasm, prostatic enlargement, neurogenic bladder, strictures, or nephrolithiasis. An alternative approach to AKI suggests that it is the balance of injurious and repair processes that may drive the rapidity of decline in kidney function in AKI.18 This molecular approach to the diagnosis and management of AKI has led to investigation of new biomarkers that detect glomerular or nephron injury earlier and provide guidance to early treatment, with a potential for improved patient outcomes.21 For example, cystatin C is emerging as a potential marker for the early diagnosis of AKI and CKD and may be useful in determining if management is contributing to improvement such as return to baseline kidney function (i.e., reversal of AKI) or stability with cessation of progression of CKD.1,2 Figure 149-1 illustrates the risk for progression of CKD. CKD and AKI have few symptoms in the early stages. Ongoing progression of CKD and destruction of nephrons result in a variety of symptoms, complications, and a steady and predictable decline in functional health. ESRD and its management (dialysis or kidney transplantation) have significant complications that need to be co-managed with nephrologists and other experts. The hallmark clinical signs of CKD and AKI are a decreased GFR, an increased serum creatinine, and albumin in the urine. The clinical symptoms are subtle and uncommon, with a GFR above 35 mL/min/1.73 m2. Therefore, suspicion for kidney disease should be based on recognition of the risk for kidney damage, particularly in patients with diabetes mellitus and hypertension. Because diabetes and hypertension are highly associated with CKD, clinical guidelines for management of both diabetes and hypertension recommend regular urinalysis with a calculation of the urinary albumin/creatinine ratio (ACR) to allow for early detection of kidney damage.13,19 Early detection of renal disease—for both CKD and AKI—is advocated to slow progression or kidney damage and decrease complications from uremia and disrupted metabolism. The most common cause of AKI is hospitalization with concomitant reduced renal perfusion and exposure to nephrotoxins.12 Most patients who develop AKI have identifiable risk factors, such as CKD, advanced age, liver disease, diabetes, or vascular disease. Therefore it is essential to identify those at high risk before AKI develops. It is equally important to follow up with monitoring after a diagnosis of AKI because patients with AKI are at risk for developing CKD in the future and may be discharged with early-stage CKD.20 Once the GFR falls below 35 mL/min/1.73 m2, a variety of cardiovascular, gastrointestinal, neurologic, metabolic, hematologic, and psychosocial problems occur. These complications are listed in Box 149-1. Clinical presentation at this point depends on the particular complication and on the underlying cause of kidney failure. All individuals with CKD should undergo screening for the complications of kidney disease to prevent morbidity and to establish a credible baseline for the individual.
Renal Failure
Definition and Epidemiology
Markers of kidney damage (one or more)
Albuminuria (AER ≥30 mg/24 hr; ACR ≥30 mg/g [≥3 mg/mmol])
Urine sediment abnormalities
Electrolyte and other abnormalities resulting from tubular disorders
Abnormalities detected by histology
Structure abnormalities detected by imaging
History of kidney transplantation
Decreased GFR
GFR <60 mL/min/1.73 m2 (GFR categories G3a-G5)
GFR Category
GFR (mL/min/1.73 m2)
Terms
G1
≥90
Normal or high
G2
60-89
Mildly decreased*
G3a
45-59
Mildly to moderately decreased
G3b
30-44
Moderately to severely decreased
G4
15-29
Severely decreased
G5
<15
Kidney failure
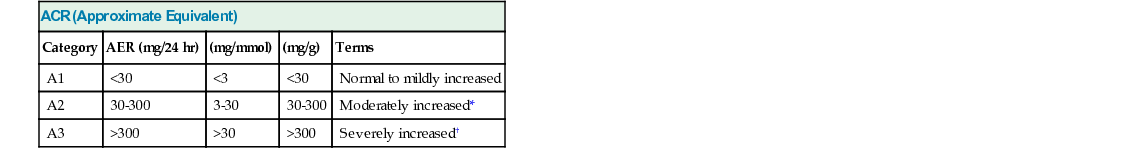
ACR (Approximate Equivalent)
Category
AER (mg/24 hr)
(mg/mmol)
(mg/g)
Terms
A1
<30
<3
<30
Normal to mildly increased
A2
30-300
3-30
30-300
Moderately increased*
A3
>300
>30
>300
Severely increased†
Stage
Serum Creatinine
Urine Output
1
1.5-1.9 times baseline
or
>0.3 mg/dL (>26 µmol/L) increase
<0.5 mL/kg/hr for 6-12 hr
2
2.0-2.9 times baseline
<0.5 mL/kg/hr for >12 hr
3
3.0 times baseline
or
Increase in serum creatinine to >4.0 mg/dL (>353.6 µmol/L)
or
Initiation of renal replacement therapy
or
In patients <18 years, decrease in estimated GFR to <35 mL/min/1.73 m2
<0.3 mL/kg/hr for >24 hr
or
Anuria for >12 hr
Pathophysiology
Clinical Presentation
Renal Failure
Chapter 149