C. Assessment of renal function during pregnancy
1. Renal function is commonly assessed by two techniques. The first measures serum levels of creatinine and BUN. However, these serum values will not increase until approximately 60% of renal function is lost. The second technique measures the loss of solute in the urine over time. The solute concentration is used to calculate GFR [(urine concentration of solute × urine flow) / (plasma concentration of solute)]. The GFR is a sensitive indicator of renal function. GFR increases 40% to 50% in normal pregnancy. A GFR and serum creatinine value considered normal in the nonpregnant population may represent renal function 40% to 50% of normal in the parturient.10
2. Many studies use serum creatinine to calculate GFR and renal function using an equation that includes age and body mass. However, creatinine clearance does not always correlate with GFR, and this technique requires a timed urine collection.
3. Serum markers used in research (e.g., cystatin C) require further testing in pregnancy before they can be used to routinely measure renal function. Placental production of cystatin C, in addition to selective decreased glomerular filtration of cystatin C, affects this test’s accuracy during pregnancy. Therefore, creatinine clearance by 24-hour urine collection is the best method to calculate GFR during pregnancy.10
4. Prediction formulas. Modification of Diet in Renal Disease (MDRD) underestimates GFR during pregnancy and is less accurate than creatinine clearance. It is not recommended for use as a screening test for renal disease during pregnancy.11
CLINICAL PEARLSerum creatinine will not increase until approximately 60% of renal function is lost.
D. Categories of renal dysfunction and influence on pregnancy
1. The severity of renal disease is categorized by the GFR.12 The GFR is quoted in the units of mL/minute/1.73 m 2 to normalize to body mass index. The five stages of renal disease are shown in Table. 27.2. A referral to a nephrologist is recommended when i) GFR is <30 mL per minute, ii) a urine–creatinine ratio >60 mg per mmol, or iii) proteinuria >1 g per day.13
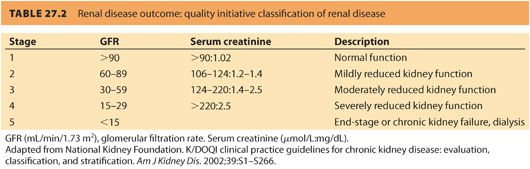
CLINICAL PEARLThe severity of renal disease is categorized by the GFR.
a. Preserved renal function with no hypertension is associated with GFR >90 (approximate serum creatinine levels of <125 μmol per L or <1.4 mg per dL). Pregnancy has a minimal effect on renal function and is not independently associated with postpartum deterioration or development of end-stage renal disease (ESRD).
b. Mild renal impairment with a GFR of 60 to 89 (approximate serum creatinine levels between 125 and 170 μmol per L or >1.4 mg per dL) is associated with minimal symptoms during pregnancy and postpartum.14
c. Moderate renal impairment with a GFR of 30 to 59 (serum creatinine approximately between 170 and 220 μmol per L or >2.4 mg per dL) is usually associated with hypertension. Postpartum loss of renal function is accelerated when moderate renal failure is associated with proteinuria of >1 g per day.15
d. Severe renal impairment is defined by a GFR between 15 and 29 (approximate creatinine >265 μmol per L or >3.0 mg per dL). Patients with hypertensive disorders associated with pregnancy are at risk for loss of renal function. In one study, women with hypertensive disorders in pregnancy had a risk of ESRD that was 11 times greater than women without hypertension. The risk was highest in women with a history of preeclampsia superimposed on chronic hypertension. Women with gestational hypertension also had a higher risk of ESRD than did women without hypertensive disorders in pregnancy. Fetal mortality was increased and low birth weight common.16
e. Patients with end-stage, dialysis-dependent renal failure often have amenorrhea and infertility. Only 1% to 7% of women with established renal failure become pregnant, and less than half will reach full-term gestation, most ending in abortion. Dialysis parameters may need adjustment during pregnancy due to the change in intravascular volume and electrolytes. Erythropoietin dose requirements for treatment of anemia are also increased. Table 27.2 reviews the “Renal Disease Outcome Quality Initiative” classification of renal disease.
CLINICAL PEARLOnly 1% to 7% of women with established renal failure become pregnant, and less than half will reach full-term gestation, most ending in abortion.
2. Patients with ESRD can have unstable blood pressures. Hypertension and rapid changes in intravascular volume and electrolytes are common during dialysis. Maternal hypotension is a common complication of hemodialysis. This can cause changes in the pulsatility index of the umbilical artery, leading to nonreassuring fetal heart rate patterns and urgent CD.
3. Pregnancy outcome and end-stage renal disease
Physiologic changes caused by ESRD contribute to an increase in fetal morbidity and mortality. The rapid changes in electrolytes, acid–base abnormalities, intravascular volume, anemia of chronic disease, and the need for anticoagulation are associated with increased risk of maternal mortality and fetal loss. Other complications of chronic renal disease (CRD) during pregnancy include intrauterine growth restriction (IUGR), preterm delivery, low birth weight, and stillbirth.
E. Systemic effects of renal disease and prognosis
CRD in pregnancy is uncommon and occurs in only 0.03% to 0.12% of parturients.
1. Hypertension tends to worsen and preeclampsia develops in 10% of these women.
CLINICAL PEARLHypertension is associated with an increased risk of abruption, peripartum bleeding, and anemia.
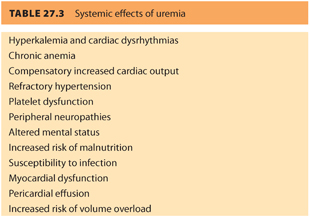
2. Uremia has many negative systemic effects (see Table. 27.3).
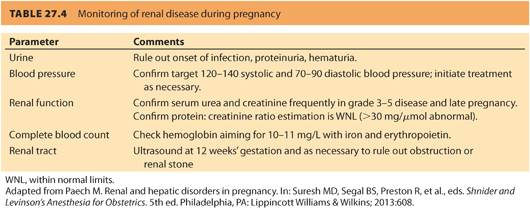
3. The two most important factors affecting prognosis are the degree of renal dysfunction and the presence of hypertension at conception. Pregnancy does not appear to accelerate the loss of kidney function or affect fetal outcome if (a) the GFR is >40 and there is <1 g per day proteinuria and (b) the patient does not have poorly controlled hypertension or other risk factors for renal failure.17 Renal function and blood pressure should be monitored during pregnancy (see Table. 27.4).
CLINICAL PEARLPatients with ESRD can have unstable blood pressures. Hypertension and rapid changes in intravascular volume and electrolytes are common during dialysis. Maternal hypotension is a common complication of hemodialysis.
F.Etiology of renal disease
1. Primary renal diseases
Two common groups of renal disorders are (a) glomerular and (b) tubulointerstitial diseases. Patients with both disorders often demonstrate proteinuria and microscopic hematuria. Although they can remain asymptomatic for many years, these disorders are responsible for most cases of chronic renal failure.
a. Glomerular diseases are a group of disorders with varied pathologies. Usually, the microvascular network of the renal cortex that ultrafiltrates blood is damaged by drugs, toxins, or the deposition of antigen–antibody complexes. Therefore, autoimmune diseases such as SLE are common causes of glomerular disease. This can cause a nephritic syndrome producing glomerular inflammatory or necrotizing lesions. Nephritic syndrome is characterized by microscopic debris of red and white blood cells and cortical cells in the urinary sediment. Protein accumulation in the glomeruli from diseases such as amyloidosis or diabetes mellitus can cause a different presentation of renal disease called nephrotic syndrome. Although the primary abnormality involves an increased loss of protein through the glomerular vessels rather than cellular debris, there is considerable overlap between nephritic and nephrotic syndromes.
b. Tubulointerstitial diseases
Renal glomeruli ultrafiltrates blood, whereas renal tubules modify the filtered fluid via resorption and secretion of various molecules. These specific actions determine the final composition of the urine. The renal tubules can be selectively affected by a variety of diseases. In general, urine concentration and composition are abnormal in tubular disease but GFR is preserved until late in the course of the disease. These diseases are commonly characterized by electrolyte imbalance.
CLINICAL PEARLPatients with glomerular and tubulointerstitial diseases often demonstrate proteinuria and microscopic hematuria.
2. Diabetes mellitus and hypertension can produce renal dysfunction in pregnancy. Diabetes is the most common cause of ESRD in the United States. Renal failure develops in up to 25% to 30% of women with type 2 diabetes mellitus after 15 years of disease. African Americans, Hispanics, and Native Americans with diabetes are at greatest risk for renal failure. The rate of gestational diabetes increases with maternal age, preexisting hypertension, urinary tract infection, and multiple pregnancies. Furthermore, the risk of preeclampsia, urinary tract infection, premature delivery, liver disease, and chronic renal disease is greater in parturients with gestational diabetes and preexisting diabetes. Preexisting diabetes and gestational diabetes are associated with increases in pregnancy-related complications, longer hospital days, and medical cost. The risk of venous thromboembolism, peripartum hemorrhage, shoulder dystocia, and placental abnormalities are greater in parturients with preexisting diabetes but not in parturients with gestational diabetes.18 Pregnancy does not affect the progression of diabetic nephropathy provided the GFR is >40. However, there is an increased risk of infection and preeclampsia.19
CLINICAL PEARLHypertension and diabetes are two systemic diseases that can produce renal dysfunction.
3. Collagen vascular diseases (e.g., SLE, rheumatoid arthritis, and scleroderma) are associated with pregnancy-related renal disease. SLE is the most common collagen vascular disease in pregnancy with an incidence of 1 in 1,660. In general, pregnancy does not increase the severity of SLE or other collagen vascular diseases, although there may be some increase in disease activity during the first month of pregnancy. Koh et al.20 noted that pregnancies with preexisting lupus nephritis had a greater frequency of adverse obstetric outcomes and maternal comorbidity. Renal flares (an increase in serum creatinine or urine protein) occurred in 50% of pregnancies with preexisting lupus nephritis, 90% of which were reactivations. Active preexisting lupus neprhitis and eGFR <90 mL/minute/1.73 m2 prior to pregnancy are associated with renal flares during pregnancy. Persistent lupus nephritis 1 year after delivery occurred in 33.3% of pregnancies, and chronic renal disease occurred in 20% of pregnancies with renal flare. There is no reason why women with SLE should avoid pregnancy unless they have advanced end-organ damage. The key to successful outcome is careful monitoring.20
4. Renal transplant patients benefit from preconception planning to discuss specific steps that will improve the likelihood of a successful pregnancy outcome. ESRD results in menstrual irregularities, anovulation, and infertility. Kidney transplantation increases fertility in patients with chronic renal disease. However, assisted reproductive technologies may be required for patients with persistent luteal insufficiency and premature ovarian failure syndrome.21 The National Kidney Foundation suggests that patients wait at least 1 year between the time of transplantation and pregnancy. Pregnancy has no deleterious effect on graft function.22 The incidence of acute renal rejection during pregnancy is 3% to 14%. However, pregnancy does not increase the risk of renal graft loss as long as patients are maintained on their antirejection drugs. Although most antirejection medications do not seem to have a negative effect on the fetus, mycophenolate, mofetil (Cellcept), and rapamycin (Sirolimus) should be discontinued at least 6 months before conception. Changes in blood volume and metabolism during pregnancy and in the postpartum period may affect the blood levels of antirejection medication and increase the risk of acute rejection. This may require adjustments in the dose or type of immune suppressants administered. Localization of the graft in the pelvis does not preclude the possibility of vaginal delivery. The incidence of prematurity and malformations (e.g., cleft lip/palate, cardiac abnormalities, congenital hydronephrosis, and undescended testicles) are increased, and perinatal mortality is 2% to 5%.
CLINICAL PEARLRenal transplant patients benefit from preconception planning to discuss specific steps that will improve the likelihood of a successful pregnancy outcome.
G. General management strategies
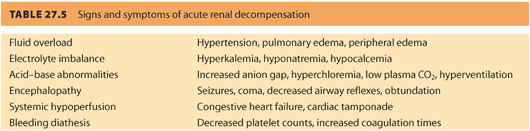
1. The primary goal for the anesthesiologist is primum non nocere that means “first do no harm,” which also applies when caring for parturients with borderline renal function or CRD. There are many conditions that can precipitate acute kidney injury (AKI) in obstetric patients with renal dysfunction. These conditions should be quickly and aggressively treated to ensure better outcomes. It is imperative to treat complications that can cause renal injury (e.g., hypotension, hemorrhage, hypovolemia, sepsis). Timely restoration of renal perfusion and renal function will help limit the extent of injury and prevent irreversible renal failure. Table. 27.5 reviews the signs and symptoms of acute renal decompensation.
2. The use of loop diuretics does not reduce maternal mortality by converting oliguric to nonoliguric AKI and is reserved for cases of volume overload. Anemia is usually well tolerated due to a shift in the oxyhemoglobin dissociation curve favoring oxygen delivery. However, anemia, hyperkalemia, and metabolic acidosis should be treated, particularly if clinical signs of uremia, such as pulmonary edema, pericarditis, neuropathy, and uremic encephalopathy, are present. Kidney function is best preserved by maintenance of euvolemia, normal blood pressure, and by avoiding renal toxins.23 Therapeutic use of osmotic diuretics for renal failure is associated with decreases in GFR and maintenance of urine flow by increasing proximal tubular water reabsorption and decreasing urinary excretion. The potential toxic effects of osmotic diuretics include increased intravascular fluid volume, hypersensitivity reactions, hyperglycemia, and glycosuria. All preclude the use of osmotic diuretics except when indicated for reduction in CSF and intraocular pressure and CSF volume during pregnancy. Additional side effects may include headaches, nausea, and vomiting.
CLINICAL PEARLThe use of loop diuretics does not reduce maternal mortality by converting oliguric to nonoliguric AKI and is reserved for cases of volume overload.
H. Anesthetic implications of renal disease
1. Altered responses to drugs
Patients with CRD may have altered responses to medications.
a. The volume of distribution of water-soluble drugs can change when intravascular volume is adjusted during dialysis. A fall in serum proteins can increase the active concentration of protein-bound drugs, and the volume of distribution of lipid-soluble drugs is often less. Furthermore, there are changes in central nervous system receptors and drug metabolism that can increase the response to sedatives, hypnotics, and analgesics. The changes in drug distribution and activity may result in hypoventilation and an inability of the patient to protect the airway. All patients with renal failure have gastroparesis and aspiration risk.
b. Serum concentration of drugs, which are excreted through the kidneys, such as magnesium sulfate, should be monitored frequently and maintenance infusion rates lowered when necessary. Drugs that are eliminated by the kidney can have prolonged action because their clearance is decreased by systemic acidosis and alterations in the volume of distribution. The loading dose of many drugs is not changed, but maintenance doses are reduced. This explains why the dose of etomidate and propofol appear to be unchanged by renal failure. However, benzodiazepines are highly protein bound and may have an increased effect when the serum protein is reduced. The initial dose of such medications should be decreased. In addition, active metabolites can rapidly accumulate with repeat doses in patients with moderate to severe renal failure.
c. Prolonged respiratory depression may be avoided with the use of shorter acting opioids that do not require renal elimination (e.g., fentanyl, remifentanil).
d. Opioids, barbiturates, propofol, and benzodiazepines are associated with mild reductions in GFR. Transplant patients are at risk for hypertension, diabetes, CD, preterm labor, and anemia. Despite this, one recent observational study noted there were no anesthetic complications associated with either general or neuraxial anesthesia.24
e. When utilizing neuraxial techniques, sterile technique should be maintained and hypotension should be treated with vasopressors to maintain renal perfusion.24
CLINICAL PEARLPatients with chronic renal disease may have changes in central nervous system receptors and drug metabolism that can increase the response to sedatives, hypnotics, and analgesics. The changes in drug distribution and activity may result in hypoventilation and an inability of the patient to protect the airway.
2. Potential nephrotoxins
a. There are several potential nephrotoxins that should be used with caution in parturients with renal insufficiency (e.g., nonsteroidal anti-inflammatory drugs [NSAIDs], aminoglycosides, anticholinergics, radiocontrast agents). NSAIDs, in particular, reduce RBF and should be used with caution in patients with renal dysfunction.
b. Some inhaled anesthetics (e.g., sevoflurane) increase inorganic fluoride levels. Although sevoflurane is metabolized to the nephrotoxic compound A, this has not been found to be clinically significant.
3. Preoperative preparation and laboratory studies
a. Preoperative evaluation of a parturient with preexisting renal disease should include a thorough history and physical examination, paying particular attention to complications associated with renal disease and any signs or symptoms of inadequate dialysis, such as uremia and fluid overload. Table. 27.6 reviews the effects of chronic renal disease on organ systems.
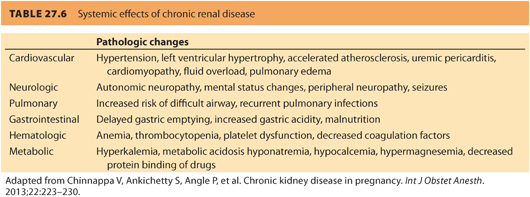
b. Laboratory studies should focus on the detection of anemia, abnormalities of coagulation and electrolytes, and urinary infection. Therefore, it is reasonable to obtain a complete blood count, coagulation profile, and electrolyte panel in all patients with renal disease. The serum magnesium level can be high in patients with renal disease. A routine magnesium level may be indicated in this patient population. Routine urinalysis may detect proteinuria associated with preeclampsia, progression of renal disease or previously undetected chronic renal disease, hematuria, and urinary tract infection (see Table. 27.4).
c. Preoperative preparation should include adequate IV access and judicious hydration. Aspiration prophylaxis should be consistent with the American Society of Anesthesiologists’ (ASA)25 Practice Guidelines for Obstetric Anesthesia. Besides routine monitoring, fetal heart tones should also be monitored. Invasive monitoring is reserved for cases with severe renal disease, worsening renal function, and hypertension. Extremities with hemodialysis fistulas should be padded carefully to prevent thrombosis. Blood pressure cuffs should not be placed on these extremities.
CLINICAL PEARLLaboratory studies should focus on the detection of anemia, abnormalities of coagulation and electrolytes, and urinary tract infection.
4. Anesthetic management
Anesthetic management aims to optimize the medical condition of the patient before labor and delivery. This includes balancing intravascular volume as well as avoiding hypotension, renal toxic drugs, and anesthetic complications. The advantages of neuraxial anesthesia have been discussed previously (see Chapter 12). Although neuraxial anesthesia offers many advantages for labor and delivery, the decision to use a particular anesthetic technique should be based on the relative risks and benefits. Spinal anesthesia has been used successfully in parturients with chronic renal disease with normal coagulation profile for elective CD.26 Relative contraindications to neuraxial anesthesia may include volume contraction due to dialysis, hypotension, and coagulation abnormalities. Serum potassium should be determined before induction of general anesthesia in patients with renal failure. Succinylcholine administration increases the serum potassium level by as much as 0.7 mEq per L. If a patient is already hyperkalemic, further elevations in potassium could cause asystole or ventricular dysrhythmia.
CLINICAL PEARLRelative contraindications to neuraxial anesthesia may include volume contraction due to dialysis, hypotension, and coagulation abnormalities.
I.Renal failure associated with pregnancy
1. Women with chronic renal disease may be unable to adapt to the increases in intravascular volume and the increased demand for excretion of waste products by the mother and fetus. These demands may exceed the capacity of the maternal kidney to provide homeostasis and may cause a decline in preexisting renal function or precipitate ARF. Renal dysfunction may be covert until the demands of pregnancy exceed the capacity of the kidney.
2. Acute kidney injury during pregnancy
a. AKI is a precipitous decline in renal function that is measured by a decrease in urine output and/or GFR.27 AKI occurs rarely during pregnancy with an incidence of 1 in 10,000 but is associated with a high mortality.28 The term acute renal failure is no longer used because it does not capture the marked increase in mortality associated with modest increases in creatinine or declines in GFR. Therefore, a working definition of AKI was developed so that physicians could detect and treat renal injury at an early stage.29 Because early intervention improves outcome, it is important that a definition of AKI is sensitive and multifaceted. The aim is to identify patients at risk for renal injury in addition to those who have established renal failure. With this task, the Acute Dialysis Quality Initiative (ADQI) developed the RIFLE classification of AKI (kidney Risk, Injury, Failure, and Loss and End-stage renal failure). This classification uses diagnostic definitions for stages of AKI, which can be treated (risk), stages of renal damage (injury), and established renal failure (loss and failure). The RIFLE score has been tested clinically in a wide variety of patient populations and has been shown to be predictive of renal outcome.
b. An increased risk of renal failure is defined by i) an increase of serum creatinine of 50% and ii) a decrease in GFR, relative to baseline, of >25% or iii) a urine output of <0.5 mL/kg/hour for >6 hours. Outcome studies show that these simple criteria can distinguish between patients who have temporary inadequate renal perfusion compared to those who have the early stages of kidney injury. Kidney injury is defined by a doubling of serum creatinine or a urinary output below 0.5 mL/kg/hour for at least 12 hours. More than 50% of patients who develop injury according to these criteria will progress to develop established renal failure.30
CLINICAL PEARLRisk of renal injury is defined by the following criteria: (a) an increase of serum creatinine by 50% and (b) a decrease in GFR, relative to baseline, of >25% or (c) a urine output of <0.5 mL/kg/hour for >6 hours.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








