RENAL AND ELECTROLYTE EMERGENCIES
REBEKAH A. BURNS, MD AND RON L. KAPLAN, MD
GOALS OF EMERGENCY CARE
Fluid imbalances, electrolyte abnormalities, and renal insufficiency are associated with a wide variety of conditions encountered in the pediatric ED. Severe derangements of electrolytes and renal function can lead to significant morbidity and mortality. Prompt recognition and appropriate management is necessary to ensure adequate circulating volume and prevent serious sequelae related to electrolyte and acid–base disturbances. Overly rapid correction of some electrolyte abnormalities may result in devastating consequences, so care must be taken in the management of these conditions. Patients with renal insufficiency may present with overall fluid overload but intravascular depletion, making fluid resuscitation challenging.
KEY POINTS
 Severe hypovolemia must be treated rapidly with intravenous boluses of isotonic saline.
Severe hypovolemia must be treated rapidly with intravenous boluses of isotonic saline.
 Once circulating volume is adequate, further treatment of hypovolemia will depend on the serum sodium.
Once circulating volume is adequate, further treatment of hypovolemia will depend on the serum sodium.
 Overly rapid correction of hypo- or hypernatremia can lead to serious CNS complications.
Overly rapid correction of hypo- or hypernatremia can lead to serious CNS complications.
 Treatment of severe hyperkalemia is aimed at stabilizing the myocardium to prevent arrhythmias and enhancing movement of potassium into the intracellular space.
Treatment of severe hyperkalemia is aimed at stabilizing the myocardium to prevent arrhythmias and enhancing movement of potassium into the intracellular space.
 Metabolic acidosis is primarily treated by attempting to correct the underlying cause.
Metabolic acidosis is primarily treated by attempting to correct the underlying cause.
 Acute kidney injury may lead to severe fluid and electrolyte disturbances that require emergent intervention regardless of the underlying etiology.
Acute kidney injury may lead to severe fluid and electrolyte disturbances that require emergent intervention regardless of the underlying etiology.
 The management of many causes of acute kidney injury is supportive in nature.
The management of many causes of acute kidney injury is supportive in nature.
 Nephrotic syndrome is often steroid responsive in children.
Nephrotic syndrome is often steroid responsive in children.
 Chronic kidney disease may go unrecognized prior to presentation to the emergency department.
Chronic kidney disease may go unrecognized prior to presentation to the emergency department.
RELATED CHAPTERS
Signs and Symptoms
• Abdominal Distension: Chapter 7
• Rash: Papulosquamous Eruptions: Chapter 65
• Respiratory Distress: Chapter 66
• Urinary Frequency: Chapter 74
• Dermatologic Urgencies and Emergencies: Chapter 96
Medical, Surgical, and Trauma Emergencies
• Endocrine Emergencies: Chapter 97
• Gastrointestinal Emergencies: Chapter 99
• Hematologic Emergencies: Chapter 101
• Metabolic Emergencies: Chapter 103
• Rheumatologic Emergencies: Chapter 109
• Abdominal Trauma: Chapter 111
• Genitourinary Trauma: Chapter 116
• Genitourinary Emergencies: Chapter 127
DEHYDRATION/HYPOVOLEMIA
CLINICAL PEARLS AND PITFALLS
• Oral rehydration is the treatment of choice for mild to moderate dehydration.
• In moderate to severe hypovolemia, isotonic crystalloid should be given intravenously in 20 mL per kg boluses until intravascular volume has been restored.
• Subsequent volume repletion strategies will be determined by serum sodium levels.
Current Evidence
Volume depletion occurs frequently in children and is a common finding in patients presenting to the emergency department. Hypovolemia refers to a decrease in the effective circulating volume, which can occur with salt and water loss or water loss alone. By definition, the term dehydration refers to water loss alone, but the terms hypovolemia and dehydration have been used interchangeably in the clinical literature. Children are at greater risk for hypovolemia than adults due to several factors: Gastroenteritis with significant volume loss occurs at a higher frequency in children; children have a higher surface area-to-volume ratio resulting in greater insensible losses; and children may be less able to access adequate fluids to replenish losses given their developmental limitations.
Goals of Treatment
Hypovolemia leads to a reduction in the effective circulating volume, which may compromise tissue and organ perfusion. Significant hypovolemia must be recognized and corrected rapidly in order to prevent hypoperfusion and ischemic end-organ damage and progression to hypovolemic shock, which is associated with significant morbidity and mortality. Fluid therapy is aimed at correcting existing abnormalities and maintaining normal volume and composition of body fluids. Hypovolemia may be associated with other electrolyte abnormalities or acid–base disturbances. Specific treatment will depend on associated abnormalities, particularly hyponatremia or hypernatremia.
Clinical Considerations
Clinical recognition. Concern for volume depletion should be raised in any patient presenting to the emergency department with a history of increased fluid losses or poor oral intake. Young children or children with developmental delay may also be at increased risk due to inability to communicate their needs and lack of access to fluid intake in response to thirst.
Triage considerations. Children with a history or appearance suggestive of hypovolemia should be assessed in a timely manner to evaluate their degree of hypovolemia and potential need for rapid intervention. While oral rehydration therapy may be appropriate for mild to moderate dehydration, children with severe hypovolemia require rapid resuscitation with intravenous isotonic crystalloid.
Clinical assessment. The initial assessment of a child with hypovolemia should include a medical history and thorough physical examination. A careful history should establish the cause of hypovolemia, duration of illness, approximate volume and composition of fluid taken in as well as urine output. Potential causes of increased insensible losses, such as fever and tachypnea, should be considered.
The physical assessment should include an accurate weight. A change in weight from a recent healthy baseline, if available, would provide the most accurate objective account of the degree of depletion. Assessment of intravascular volume should include the pulse quality and rate, blood pressure, hydration of mucous membranes, skin turgor and perfusion, mental status, and activity. Mild hypovolemia (3% to 5% volume loss) may be associated with minimal or absent clinical signs. Moderate hypovolemia (6% to 9% volume loss) will have clinical signs apparent, which may include tachycardia, orthostatic blood pressure changes, dry mucous membranes, and delayed capillary refill time. Several dehydration scores (Gorelick score, WHO score, and clinical dehydration score) have been proposed to aid in estimating degree of dehydration based on clinical findings, with mixed results. A systematic review of published data reported by Steiner et al. revealed that the most useful individual signs for predicting 5% hypovolemia in children were delayed capillary refill time, abnormal skin turgor, and abnormal respiratory pattern. A combination of examination signs provided the best predictive data. In the setting of severe dehydration (greater than or equal to 10% volume loss), evidence of shock may be apparent, with hypotension, poor peripheral perfusion with prolonged capillary refill time, cool or mottled extremities, lethargy, and rapid deep respirations. Severe hypovolemia requires immediate attention with aggressive isotonic fluid resuscitation (see Chapter 5 Shock).
Though laboratory assessment has been shown to be less useful than physical findings when predicting the degree of volume depletion, laboratory testing can identify associated electrolyte and acid–base abnormalities. Classification of the type of hypovolemia based upon the serum sodium may impact subsequent fluid therapy and monitoring. Solute is primarily composed of sodium salts in the extracellular fluid (ECF) and potassium salts in the intracellular fluid (ICF). The presenting serum sodium in the child with hypovolemia results from the loss of solute relative to water during the illness. Determinants of the serum sodium include the type of fluid lost, the composition of fluid provided prior to presentation, and the ability to excrete water during the illness.
Hyponatremic hypovolemia (serum sodium less than 135 mEq per L) reflects the net loss of solute in excess of water. Isonatremic hypovolemia (serum sodium 135 to 145 mEq per L) results when solute is lost in proportion to water, and hypernatremic hypovolemia (serum sodium greater than 145 mEq per L) reflects net loss of water in excess of solute.
Other biochemical abnormalities that may develop during hypovolemia include disorders of potassium homeostasis, acid–base abnormalities, and increased blood urea nitrogen and creatinine, reflecting a decline in glomerular filtration rate (GFR). Though hyperkalemia may result, hypokalemia is more commonly seen in children with gastroenteritis given the loss of potassium in diarrheal fluid and urine. Urine losses of potassium may be significant and driven by aldosterone. The effect of aldosterone is to conserve urinary sodium to maintain effective intravascular volume and promote potassium excretion.
Management. Initial management will depend on the severity of hypovolemia and presence of abnormalities of serum sodium, but the aims of treatment are to restore perfusion and maintain adequate volume in the face of ongoing losses. Oral therapy, when tolerated, is the preferred treatment of fluid and electrolyte losses in children with mild to moderate dehydration. In general, limitations to ORT include severe dehydration, altered mental status, possible surgical pathology that would mandate NPO status, abdominal ileus or disorders that limit intestinal absorption, severe and persistent vomiting, excessive stool losses, and severe electrolyte abnormalities.
Patients who have moderate or severe hypovolemia will have compromised effective circulating volume, and rapid volume resuscitation is required to restore perfusion and avoid tissue damage. Emergent intravenous fluid therapy should be provided with a rapid infusion of 20 mL per kg of isotonic crystalloid. 0.9% sodium chloride (NS) is used most frequently, but lactated Ringer (LR) solution may be appropriate as well. Patients with severe hypovolemia should receive their first bolus over 20 minutes by pressure bag or push–pull technique, and may require up to 60 mL per kg in the first hour of resuscitation (see Chapter 5 Shock). There is currently no evidence that addition of dextrose to the crystalloid provides any significant clinical benefit without evidence of hypoglycemia. The child should be reassessed during and at completion of the intravenous bolus in order to determine if additional bolus therapy is indicated. Crystalloid boluses should be repeated until adequate perfusion has been restored. Intraosseous administration of volume replacement is an appropriate alternative if intravenous access is not available. Currently, there are inadequate data to support the use of colloid-containing solutions during resuscitation in the general population. However, if a patient with decreased oncotic pressure due to an illness such as nephrotic syndrome or cirrhosis presents with hypovolemia, he may benefit from infusion of a colloid solution such as 5% albumin when serum albumin level is less than 2.0 to 2.5 g per dL.
TABLE 108.1
WEIGHT-BASED DAILY MAINTENANCE FLUID FOR CHILDREN

Once circulating volume has been adequately restored, the second phase of fluid therapy corrects persistent deficits, replaces ongoing losses, and provides maintenance fluids. The maintenance requirements for fluid in children are outlined in Table 108.1. Though common practice has been to provide relatively hypotonic maintenance fluids (D5 ¼ NS or D5 ½ NS) based upon deficit calculations and maintenance requirements, recent reports have highlighted the potential risks of acquired hyponatremia in some hospitalized patients receiving hypotonic fluids (especially in the setting on substantial ongoing losses). Some have proposed that dextrose-containing isotonic fluid be continued in the ongoing repletion and maintenance phase of therapy with periodic assessment of serum Na to determine if further adjustment in fluid composition is warranted. If isotonic fluid is used for maintenance therapy, the risks of sodium excess, inadequate free-water provision during ongoing hypotonic losses, and hypernatremia must be considered. Currently there are inadequate data to support the routine use of isotonic solutions for ongoing fluid repletion and maintenance therapy in hypovolemia. Further studies are needed to compare the efficacy and safety of differing fluid regimens.
Examples of the treatment of isonatremic, hyponatremic, and hypernatremic hypovolemia are provided in Tables 108.2 through 108.4. In all cases, the fluid of choice for the initial emergent phase of volume resuscitation is isotonic saline. In most cases, the plan for replacement usually does not need to be exact as the kidneys will correct the electrolytes once well perfused and additionally enteral feeding is often initiated.
Isonatremic hypovolemia. Table 108.2 outlines the estimated deficits and therapeutic approach to a 10-kg child with isotonic hypovolemia. In this example, the isotonic deficit is corrected by an initial bolus of isotonic saline followed by ongoing repletion. The traditional approach of deficit calculation and therapy is outlined. Replacement of ongoing extrarenal losses should be provided if the volume of losses is significant. Repeated assessment of the serum sodium may be indicated on the basis of losses and duration of intravenous therapy.
Hyponatremic hypovolemia. Children with mild to moderate hyponatremia should be provided isotonic or near-isotonic fluids to both complete the repletion phase and continue the maintenance phase of intravenous fluid therapy. Providing hypotonic fluid in the setting of persistent decreased intravascular volume and acute illness with osmotic and nonosmotic stimuli for ADH secretion may perpetuate or worsen hyponatremia. Isotonic saline will correct the volume depletion and raise the serum sodium concurrently. The therapy of asymptomatic hyponatremia requires gradual correction of the serum sodium with a target increase of less than 10 to 12 mEq/L/day.
TABLE 108.2
ESTIMATED DEFICITS AND INTRAVENOUS THERAPY: 10-kg CHILD WITH 10% HYPOVOLEMIA AND SERUM SODIUM 140 mEq/L

Table 108.3 estimates the sodium and water deficits and outlines a plan for fluid management of a child with hyponatremic hypovolemia and serum sodium of 125 mEq per L. The sodium deficit is estimated by the following equation using the serum sodium concentration and estimates of total body water (TBW):
Na deficit = [TBW(n) × 140 mEq/L] − [TBW(c) × serum Na]
In this calculation, TBW(n) is the estimated normal TBW, TBW(c) is the estimated current TBW, and serum sodium is the serum sodium concentration. The normal TBW in term neonates, toddlers, and older or pubertal children is approximately 75% to 80%, 65% to 70%, and 60% of body weight, respectively.
As in the previous example, circulating volume is restored with isotonic saline, and deficits are replaced over the next 24 to 48 hours. From a practical standpoint, maintenance therapy may begin with D5 NS at one-and-a-half times maintenance, and sodium concentration of subsequent fluids adjusted based on response. Care must be taken to avoid overly rapid correction of the serum sodium to prevent severe central nervous system (CNS) sequelae associated with osmotic demyelination. The sodium level should be rechecked 2 hours after initiation of treatment and at regular intervals thereafter to monitor the rate of correction. If the rate of rise of the serum sodium is greater than targeted (10 to 12 mEq/L/day), the concentration of sodium in the intravenous fluids should be reduced. The therapy of severe hyponatremia or symptomatic hyponatremia is discussed further in the section “Hyponatremia.”
TABLE 108.3
ESTIMATED DEFICITS AND INTRAVENOUS THERAPY: 10-kg CHILD WITH 10% HYPOVOLEMIA AND SERUM SODIUM 125 mEq/L

Hypernatremic hypovolemia. In children who present with hypernatremic hypovolemia, the total fluid deficit is composed of both a free-water deficit and an isotonic deficit. A pure water deficit is consistent with dehydration. Hyperosmolality initially promotes water movement out of the cells, including brain cells. Over several days, idiogenic osmoles are generated within the brain cells, prompting water movement into the intracellular space, restoring normal brain volume. Once cerebral adaptation has occurred, rapid correction of the serum sodium can result in cerebral edema and severe neurologic consequence. The goal of therapy in children with a serum sodium concentration above 150 mEq per L is to correct the hypernatremia at a rate of less than 10 to 12 mEq per L in 24 hours. The total fluid deficit can be inferred by the estimated weight loss. Calculation of the free-water deficit is based upon the serum sodium and estimated current body water:
TABLE 108.4
ESTIMATED DEFICITS AND INTRAVENOUS THERAPY: 10-kg CHILD WITH 10% HYPOVOLEMIA AND SERUM SODIUM 155 mEq/L

Free water deficit = TBW(c) × [(serum Na/140) − 1]
The difference between the total fluid deficit and the free-water deficit is the estimated isotonic deficit. Table 108.4 estimates the sodium and water deficits and outlines a plan for fluid management of a child with hypernatremic hypovolemia and serum sodium of 155 mEq per L. After the patient has received the initial isotonic fluid bolus to emergently restore intravascular volume, subsequent therapy should correct the remaining isotonic deficit, free-water deficit, ongoing losses, and maintenance requirements. Depending on the acuity and severity of the process, the free-water deficit should be replaced gradually to allow judicious correction of the serum sodium at the desired rate. In general, D5 ¼ NS at one-and-a-half times maintenance would be expected to correct deficits over 36 to 48 hours. Given the uncertainty of any correction plan and the possibility of ongoing losses, the best approach is to measure the sodium frequently as it corrects and adjust fluid content and rate as indicated.
DISORDERS OF SODIUM HOMEOSTASIS
Goals of Treatment
Hyponatremia (serum sodium less than 135 mEq per L) and hypernatremia (serum sodium greater than 150 mEq per L) are both associated with severe sequelae, and overly aggressive treatment of each can cause significant CNS complications. Treatment corrects the sodium abnormalities using estimated volume status and total body sodium content. Severe cases need to be treated at an appropriate rate in order to prevent CNS complications.
CLINICAL PEARLS AND PITFALLS
• Hyponatremia may occur secondary to increased ADH activity or in patients with hypovolemia managed with excess free water.
• Hypernatremia is usually due to excessive water loss relative to sodium and is associated with gastrointestinal illness or systemic infection.
• Hypernatremia may occur in infants due to inadequate breast-feeding or increased sodium load from improperly mixed formula.
• Clinical manifestations of hypo- or hypernatremia depend on the severity and rate of development.
• Overly rapid correction of hypo- or hypernatremia may lead to severe CNS sequelae.
Hyponatremia
When approaching a patient with hyponatremia, it is necessary to estimate the patient’s total body sodium and water based on history and physical examination. There are numerous causes of hyponatremia (Table 108.5) associated with normal or increased total body sodium, including states of impaired water excretion such as renal failure and the syndrome of inappropriate antidiuretic hormone (SIADH) secretion. Release of ADH is associated with a number of clinical conditions, including hypovolemia, fever, CNS trauma, infections and tumors, pulmonary infections, hypothyroidism, and cortisol deficiency. Certain medications, including some chemotherapeutic agents and antiepileptic agents, can be associated with inappropriate ADH release. It is critical to assess for underlying causes associated with increased total body sodium and water, as in the edema-forming states. In these clinical circumstances, providing supplemental sodium would aggravate the state of volume excess.
TABLE 108.5
CAUSES OF HYPONATREMIA BASED UPON TOTAL BODY SODIUM CONTENT
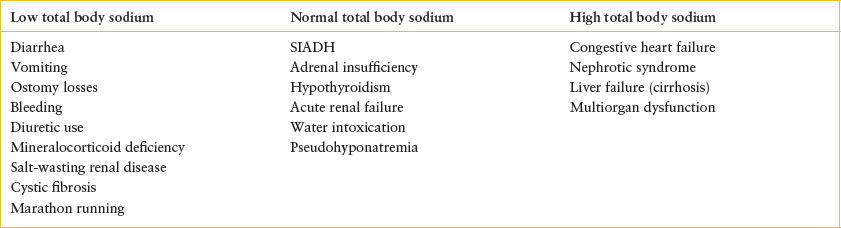
Appropriate evaluation to determine the cause of hyponatremia begins with a thorough physical examination in order to estimate volume status. History may reveal obvious sources of sodium loss or raise the concern for water intoxication. Laboratory tests should include serum electrolytes, osmolality, and assessment of renal function. Concomitant urine studies should include osmolality, urine sodium, and urinalysis. In children with hyponatremia and concentrated urine, the urine sodium may distinguish between states of decreased effective circulating volume (urine sodium <25 mEq per L) and euvolemic hyponatremia, such as the SIADH (urine sodium >40 mEq per L).
Clinical manifestations. The symptoms of hyponatremia are primarily neurologic and due to the development of cerebral edema. The symptoms mirror the severity of cerebral edema, which in turn is related to the degree of hyponatremia and the acuity of the process. The mechanisms of cellular adaptation include movement of intracellular electrolytes to the extracellular space, which can occur within minutes. Over hours to days, organic solutes move to the extracellular space. Given the ability for cerebral adaptation, the degree of cerebral edema and neurologic symptoms are less severe in chronic hyponatremia. Early neurologic symptoms include nausea and malaise, and may be seen when the serum sodium concentration falls below 125 mEq per L. With progressive derangement of cerebral cell volume, symptoms of headache, altered mental status, lethargy, ataxia, and psychosis may ensue. Signs of severe cerebral edema include seizures, coma, and respiratory depression.
Management. For children with hyponatremia associated with hypovolemia, isotonic solutions should be provided to restore intravascular volume. Children with symptomatic hyponatremia require urgent treatment to avoid progressive neurologic complications. Symptoms are more likely to develop if hyponatremia evolves rapidly, as water will move along an osmotic gradient from the extracellular space to the intracellular space. Given the effect of cell volume regulatory mechanisms, an important goal is to control the rate of rise in serum sodium to prevent rapid fluid shifts into the extracellular space and avoid the development of osmotic demyelination. The general recommendation for a child with severe hyponatremia is to increase the serum sodium no more rapidly than 12 mEq per L in the first 24 hours or an average of 0.5 mEq/L/hr. An exception to this recommendation would be symptomatic hyponatremia and evolving cerebral edema and seizures. Symptomatic hyponatremia calls for a more aggressive initial correction of the serum sodium of approximately 2 mEq/L/hr for 2 to 3 hours, which should result in clinical improvement. This can be achieved with the administration of hypertonic 3% saline (513 mEq per L of sodium). In general, 3 mL per kg of 3% saline would be expected to raise serum sodium by approximately 3 mEq per L. A practical approach is to administer doses of 3 mL per kg (maximum dose 100 mL) until seizures stop. After the initial correction is achieved, the goal for the daily correction remains approximately 12 mEq per L in the first 24 hours (including the initial emergent correction). Frequent assessment of serum sodium is necessary to avoid rapid correction, which may lead to the osmotic demyelination syndrome.
TABLE 108.6
CAUSES OF HYPERNATREMIA BASED UPON TOTAL BODY SODIUM CONTENT
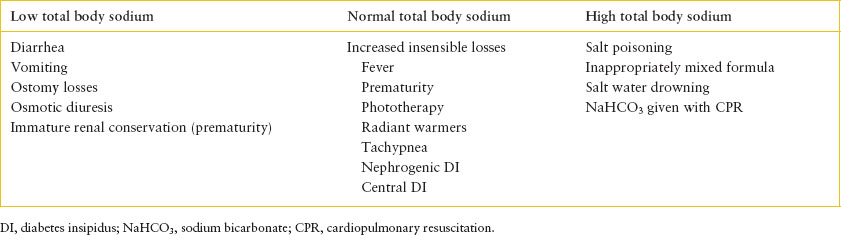
Patients who have asymptomatic hyponatremia and euvolemia do not require urgent intervention. The care of these patients should be carefully planned and based upon the underlying diagnosis with the aim of gradual correction. If hyponatremia is associated with an edema-forming state, providing supplemental sodium will worsen the state volume excess. The goal of therapy would be to achieve negative water balance in excess of negative sodium balance. To achieve this effectively, the underlying pathophysiology must be considered, although initial water restriction is generally indicated. Sodium restriction and diuretic therapy may also be warranted. The treatment of SIADH begins with water restriction, though this may be insufficient. Some cases of SIADH require the administration of salt supplements and loop diuretics to achieve the desired negative water balance, as guided by consultation with a pediatric nephrologist.
Hypernatremia
Hypernatremia can result from an increase in the total body solutes, a decrease in body water, or a reduction of body water relatively greater than a concurrent reduction in total body solutes. Protective mechanisms to prevent the development of hypernatremia include the stimulation of thirst and the ability to excrete concentrated urine, thereby minimizing free-water loss. For these mechanisms to be effective, there must be adequate access to and the ability to retain free water. Given the potential for limited access to water, infants and children with significant developmental delay are predisposed to hypernatremic dehydration. The causes of hypernatremia based on total body sodium are outlined in Table 108.6. Hypernatremia due to isolated water deficit is termed dehydration. If both salt and water deficits are present, this condition is termed hypovolemia.
Diarrhea is a common cause of hypernatremia in the acute care setting. Although the degree of sodium deficit may vary, generally children who present for care have true hypovolemia. Breast-fed infants may be at increased risk of hypernatremia due to inadequate intake. Hypernatremia due to salt excess is rare but it can occur with the improper mixing of infant formulas or iatrogenic administration of a salt load. The latter can result after sodium bicarbonate infusion during cardiopulmonary resuscitation or during therapy of refractory metabolic acidosis. Hypernatremia secondary to nearly pure water loss may develop if replacement of insensible water loss from the skin and respiratory tract is inadequate. Central diabetes insipidus is due to insufficient release of ADH from the hypothalamus, and nephrogenic diabetes insipidus is due to a renal resistance to the effect of ADH. Most children affected with these disorders have normal thirst and free access to water and are able to maintain acceptable water balance. However, infants who do not have free access to water and children with intercurrent illness precluding adequate intake of free water are at risk for the development of hypernatremic dehydration.
The cause of hypernatremia is usually evident from the presenting history. Feeding history in breast-fed infants may reveal inadequate intake. In formula-fed infants, an accurate account of formula preparation should be pursued to evaluate for inappropriate mixing, which would result in increased renal osmotic load. Inquiries of urine volume should also be made, as the production of significant urine in a child who presents with apparent hypernatremic dehydration suggests diabetes insipidus. The physical examination should assess weight, perfusion, and mental status. During hypernatremic hypovolemia, water moves from the intracellular to the extracellular space. Given the relative preservation of the extracellular volume, the objective signs of volume depletion may develop later in the patient’s course of illness. Laboratory studies should include serum electrolytes, serum osmolality, BUN, and renal serum creatinine. If the underlying diagnosis remains in question, urine studies may be informative. Urine osmolality should be compared to serum osmolality and would be elevated if renal concentrating mechanisms are intact. If the urine osmolality is inappropriately low when compared to the serum osmolality, then central or nephrogenic diabetes insipidus should be considered. The urine sodium concentration may also assist diagnosis. During hypernatremic hypovolemia, the urine sodium is generally less than 25 mEq per L due to the effect of aldosterone to maintain perfusion. If hypernatremia is due to salt excess, the appropriate renal response is to excrete sodium, and the urine sodium concentration would be elevated.
Clinical manifestations. Similar to hyponatremia, the clinical manifestations of hypernatremia are primarily neurologic. The rise in the plasma osmolality causes water movement out of the brain. The decrease in brain volume may lead to the rupture of the blood vessels contained in the membranes that tether the brain to the overlying skull and elsewhere. Early clinical manifestations can include lethargy, weakness, fever, and irritability. More severe manifestations include seizures and coma. Symptoms are more likely if the disturbance is acute and rapid. If hypernatremia evolves more slowly, there is cerebral adaptation to protect brain cell volume. Adaptive mechanisms include movement of cerebrospinal fluid into the brain, uptake of sodium and potassium into the cells, followed by intracellular accumulation of osmolytes.
Management. During sustained hypernatremia, cerebral adaptation occurs over several days to restore brain cell volume. Rapid correction of the serum sodium after cerebral adaptation occurs will result in osmotic movement of water into the brain and cerebral edema. Data suggest that the plasma sodium concentration should be lowered by less than 0.5 mEq/L/hr and no more than 12 mEq/L/day. In children with hypernatremia due to salt loading, treatment should facilitate renal excretion of sodium. This is typically achieved with salt restriction and free water and may be facilitated with diuretics. If intravenous fluids are required, the sodium plus potassium concentration of the fluid provided should be less than the sum of these electrolytes in the urine, and the fluid should be given at a rate sufficient to achieve positive water balance. Adequate intravascular volume should be assured to allow renal excretion of sodium. The therapy of children with hypernatremic hypovolemia (sodium and water deficit) was previously reviewed. In summary, children with clinical signs of decreased effective circulating volume should be provided with isotonic saline to restore perfusion. Once intravascular volume is restored, hypotonic fluids should continue to allow judicious restoration of the estimated free-water deficit, with close monitoring to avoid overly rapid correction.
DISORDERS OF POTASSIUM HOMEOSTASIS
Goals of Treatment
Potassium is the most abundant intracellular cation in the body with only approximately 2% of total body stores present in the extracellular space. Abnormalities of serum potassium are associated with abnormal neuromuscular function and risk of severe cardiac sequelae, especially with hyperkalemia. Treatment is aimed at preventing emergent cardiac complications and restoring normal serum potassium levels.
CLINICAL PEARLS AND PITFALLS
• Total body potassium depletion is present in patients with diabetic ketoacidosis (DKA) despite having normal or elevated serum potassium levels.
• Hyperkalemia should be confirmed to rule out pseudohyperkalemia due to a hemolyzed sample.
• Severe hyperkalemia may lead to cardiac arrhythmias, and initial treatment is aimed at stabilizing the myocardium with IV calcium.
• Subsequent treatment of hyperkalemia involves enhancing intracellular movement of potassium, followed by removal of excess potassium from the body.
Hypokalemia
Hypokalemia is defined as a measured serum potassium concentration below 3.5 mEq per L. Hypokalemia may result from total body deficit, transcellular shift of potassium to the intracellular space, or a combination of both processes. There are numerous causes of hypokalemia including renal loss, extrarenal loss, and increased cellular uptake, which are outlined in Table 108.7. The common causes of hypokalemia seen in pediatric emergency departments are those due to gastrointestinal loss, diuretic use, and DKA. Metabolic alkalosis will also lead to hypokalemia due to transcellular shift of potassium to the intracellular space. For every 0.1 U rise in blood pH, serum potassium would be expected to decrease by approximately 0.4 to 0.6 mEq per L.
TABLE 108.7
CAUSES OF HYPOKALEMIA
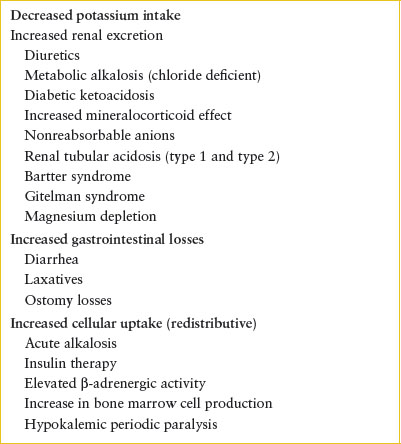
Potassium homeostasis is complex and dynamic in the setting of DKA (see Chapter 97 Endocrine Emergencies). Urinary losses of potassium are high, so patients with DKA generally have total body potassium depletion at presentation. However, the combination of insulin deficiency, hyperosmolality, and acidosis may result in normal or elevated serum potassium at presentation. Hypokalemia in a child who presents with DKA would suggest significant potassium depletion and need for supplementation with close monitoring.
The cause of hypokalemia may be inferred after a careful history is obtained. This should include a thorough account of medications taken, such as diuretics, laxatives, and beta agonists. The laboratory assessment of hypokalemia should include serum electrolytes, magnesium, calcium, serum bicarbonate, renal function, and glucose. In addition to urinalysis and urine pH, urine electrolytes and osmolality should be submitted to assess the renal response to hypokalemia.
Clinical manifestations. The clinical manifestations of hypokalemia are generally proportionate to the severity and duration of the disorder and result from hyperpolarization of the cell membrane. Unless the serum potassium falls rapidly or is associated with digitalis use, symptoms are typically not apparent until the serum level is below 2.5 mEq per L. Symptoms may also vary dependent on the concentration of other ions, including calcium, magnesium, and hydrogen. Clinically relevant signs and symptoms of hypokalemia relate to abnormal neuromuscular function and cardiovascular effects, and monitoring of muscle strength and electrocardiogram (ECG) are indicated to assess the functional consequences of hypokalemia. Neuromuscular dysfunction typically manifests as skeletal muscle weakness in an ascending pattern with worsening hypokalemia. Lower extremity muscles are initially affected with progression to the trunk and upper extremities. Respiratory weakness may develop and lead to respiratory failure. Smooth muscle dysfunction can lead to nausea, vomiting, constipation, and voiding dysfunction with urinary retention. Significant hypokalemia produces characteristic changes on the ECG. As the serum potassium drops, T-wave amplitude declines, U waves develop, and ST segment depression may result. With more profound hypokalemia the QRS complex may widen and the PR and QT intervals may prolong. Supraventricular and ventricular dysrhythmias may develop, the likelihood being greater for patients taking digitalis and in patients with congestive heart failure and coronary ischemia. Hypokalemia may also lead to an impaired ability to concentrate urine, an acquired form of nephrogenic diabetes insipidus.
Management. The therapeutic approach to hypokalemia will depend on severity, acuity, associated clinical signs, and underlying conditions. In general, potassium replacement is indicated when there has been potassium loss. If hypokalemia is redistributive in nature but associated with either severely depressed levels or clinical signs, supplementation may be required. However, supplementation during a redistributive process should proceed with close monitoring given the risk of rebound hyperkalemia. When potassium loss is accompanied by acid–base disturbance, a redistribution effect should be factored when losses are estimated. Magnesium supplementation is indicated in hypokalemia associated with hypomagnesemia. In all cases of significant hypokalemia, monitoring for ECG changes and muscle strength is imperative, and if abnormalities are present, immediate replacement is warranted.
The choice of oral or intravenous replacement will depend on the severity of the disorder and the ability to tolerate enteral salts. If the child is clinically well, oral therapy is preferable and can be provided two to four times per day as potassium chloride. Dosing may start at 2 to 5 mEq/kg/day and be adjusted on the basis of serial laboratory assessment. If there is concurrent metabolic acidosis, potassium citrate or bicarbonate can be provided. If the child is unable to take oral medications or is symptomatic, intravenous potassium should be provided. If the child is not symptomatic, potassium can be added to the maintenance fluids. If intermittent infusion is indicated, this can begin with an intravenous dose of 0.5 to 1 mEq per kg (typical maximum 30 to 40 mEq per dose). Potassium chloride is typically used, but potassium phosphate may be used in the treatment of DKA or documented severe hypophosphatemia. Potassium acetate or its equivalent may be used if there is metabolic acidosis. The infusion rate for clinically stable patients should provide 0.25 mEq/kg/hr, though emergent conditions may warrant the maximal rate of 0.5 to 1 mEq/kg/hr (maximum 15 to 40 mEq per hour depending on local policy) with continuous ECG monitoring.
Hyperkalemia
Hyperkalemia is typically defined as serum potassium concentration exceeding 6 mEq per L in neonates or 5.5 mEq per L in children or adults. Serum potassium concentration is determined by the interplay of potassium intake, distribution between the intracellular and extracellular space, and renal excretion. Hyperkalemia can therefore result from excessive load, transcellular shift, decreased renal excretion, or a combination of these factors.
If hyperkalemia develops, both endogenous and exogenous sources of potassium should be considered. Potential sources of exogenous potassium include large volume packed red blood cell transfusion, medicines with significant potassium content, and potassium salt infusions. Given improvements in blood bank procedure, hyperkalemia is less common with transfusion of red cells. Endogenous sources of potassium may result from tissue damage, including burns, trauma, rhabdomyolysis, hemolysis, tumor lysis, and gastrointestinal bleeding with enteral reabsorption. Clinical scenarios associated with extracellular shift include metabolic acidosis, hyperosmolarity, insulin deficiency, and the use of β-adrenergic receptor antagonists. Reduced renal excretion of potassium may occur in acute or chronic renal insufficiency, hypovolemia, mineralocorticoid deficiency, inherited or acquired renal tubulopathy, and due to the use of certain medications (Table 108.8).
TABLE 108.8
MEDICATIONS ASSOCIATED WITH HYPERKALEMIA

Evaluation begins with a thorough history with specific inquiries regarding injuries, muscle pain, history of renal disease, and medications taken. Serum potassium should be repeated to rule out pseudohyperkalemia, which results from a hemolyzed specimen due to difficulties in obtaining the specimen. Serum sodium, chloride, calcium, phosphorus, bicarbonate levels and measures of renal function should also be obtained. Serum creatinine kinase should be submitted if there is suspicion for rhabdomyolysis. A CBC should be obtained if there is possibility of hemolysis. Urine electrolytes and osmolality should be obtained. An ECG should be obtained to monitor for cardiac effect.
Clinical manifestations. The clinical features associated with hyperkalemia are a consequence of altered cellular transmembrane potassium gradient, which reduces the resting membrane potential. Initially this increases membrane excitability, which is followed by a sustained reduction in excitability. Unless the rise is rapid, symptoms or signs generally do not become apparent until the serum potassium concentration exceeds 7 mEq per L. Clinical features predominantly involve cardiac conduction and neuromuscular disturbance. Cardiac dysrhythmias are the most serious consequence, and toxicity is exacerbated by a rapid rise in potassium concentration, acidosis, hyponatremia, and hypocalcemia. Early ECG changes include narrow, peaked T waves with shortened QT interval, which is followed by progressive lengthening of the PR interval and widening of the QRS complex. There may be loss of P-wave amplitude and eventual “sine wave” pattern when the QRS merges with the T wave. This is typically followed by ventricular fibrillation or standstill. Neuromuscular effects are rarely evident at potassium concentrations less than 8 mEq per L and include paresthesias, skeletal muscle weakness, and ascending flaccid paralysis. Respiratory muscles are typically spared.
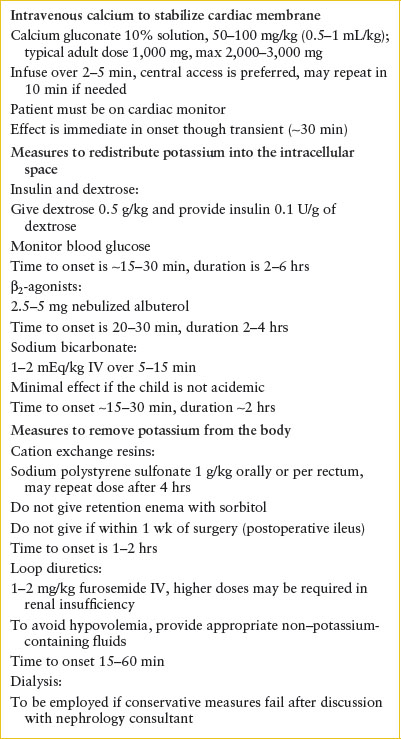
Management. Treatment of hyperkalemia includes antagonizing the cell membrane effects of hyperkalemia, shifting potassium to the intracellular space, and removing potassium from the body (Table 108.9). The urgency of care should be based upon the degree of hyperkalemia and evidence of cardiac or neuromuscular effect. Should there be ECG changes consistent with hyperkalemia, the patient should be placed on cardiac monitor and intravenous calcium should be provided to stabilize cardiac membranes. Calcium administration is indicated only in instances of significant ECG changes, such as widening of the QRS or loss of the P wave but is not indicated in isolated peaked T waves. The effect of calcium is nearly immediate but also transient and should be coupled with other measures to shift potassium to the intracellular space and remove potassium from the body. Calcium gluconate (10% solution) 50 to 100 mg per kg IV or calcium chloride (10% solution) 20 mg per kg IV is infused over 2 to 5 minutes (never pushed rapidly) with continuous cardiac monitoring. The usual adult dose of calcium gluconate is 1,000 mg and calcium chloride is 500 to 1,000 mg. Calcium is irritating to veins and can result in tissue necrosis; therefore, central vein access is preferable for both the gluconate and chloride salts and strongly recommended if calcium chloride is infused (however, do not delay calcium administration via a peripheral vein in the setting of life-threatening hyperkalemia). In patients taking digitalis, an immediate cardiac consultation should be obtained prior to administration, if possible, as calcium therapy may precipitate other dysrhythmias.
Several therapies are aimed at shifting potassium into the intracellular space and are outlined in Table 108.6. Insulin drives movement of potassium into the cell. This can be achieved by administering a combination of 0.1 units per kg of regular insulin and 0.5 g per kg of dextrose. The time of onset is 15 to 30 minutes, peak effect at approximately 60 minutes, and duration of several hours. Providing sodium bicarbonate raises the systemic pH and promotes hydrogen movement out of the cells accompanied by potassium movement into the cells. This is most effective in patients with metabolic acidosis. In the absence of acidosis, sodium bicarbonate is not routinely recommended because it is less likely to be effective, and may lead to other complications. The onset of the effect of sodium bicarbonate is within 15 to 30 minutes and persists for several hours. β2–Adrenergic agonists promote transcellular shift of potassium, so nebulized albuterol may provide further benefit. It is particularly useful if intravenous access is not available. Recommended doses of albuterol are 0.4 mg for neonates, 2.5 mg for infants and children under 25 kg, 5 mg for children 25 to 50 kg, and 10 mg for patients over 50 kg. Alternatively, albuterol may be given as 4 to 8 puffs with a metered dose inhaler with a spacer.
TABLE 108.9
THERAPIES FOR HYPERKALEMIA
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







