14 Rehabilitation and Recovery from Spinal Injuries Tom Stanley, Bart Wojewnik, Alexander R. Vaccaro, and Kern Singh Rehabilitating spinal cord injury (SCI) patients requires a multidisciplinary approach that goes far beyond restoration of physical function and medical management. SCI is a life-changing event, and the ultimate goals of postinjury rehabilitation are independence and reintegration into society. This chapter focuses on the physiologic complications of SCI and their management as well as current trends in physical rehabilitation. The National Spinal Cord Injury Database is the largest SCI registry, yet it captures only 13% of all SCIs in the United States.1 There are 11,000 new SCI cases each year, with 250,000 SCI patients currently living in the United States. Eighty percent of injuries occur in males primarily between the ages of 16 and 30. Motor vehicle accidents account for the majority of injuries, followed by falls, acts of violence, and sports. Respiratory complications are the number one cause of death in patients with spinal cord injury.2 Any injury above the level of T12 results in impaired respiratory function. Patients with injuries at functional levels below T12 are also prone to pulmonary complications from inactivity. Diminished vital capacity leads to atelectasis and pneumonia.3 Two primary areas for improving pulmonary function are cough generation and endurance training. In a prospective analysis of 187 patients, Haisma et al4 demonstrated an increase in respiratory function after rehabilitation. Alternatively, Brooks et al5 did not find any clear evidence that rehabilitation improved respiratory function. Some authors believe that focusing on cough generation helps prevent the majority of respiratory complications.6 Future research needs to address the use of epidural spinal cord stimulators to induce coughing; however, these devices are still being fully developed.7 Although pulmonary complications represent the number one cause of death in all SCI patients, patients surviving longer than 30 years and those older than 60 years of age die primarily from cardiac complications.2,8,9 SCI has both acute and chronic effects on the cardiovascular system. Spinal cord injuries above the T6 level affect sympathetic outflow and cause autonomic dysfunction, leading to episodes of profound hypotension or hypertension.10,11 A specific condition termed autonomic dysreflexia results in a loss of sympathetic inhibition to noxious stimuli, leading to vasoconstriction, hypertension, and headache. Urgent treatment is required to reduce intracranial pressure and prevent stroke. Decreased venous return from the nonfunctional lower extremities results in decreased stroke volume and increased resting heart rate. Long-term effects include left ventricular hypertrophy and cardiovascular collapse. Spinal cord injury patients are prone to developing DVT and pulmonary embolism secondary to immobility and venous stasis. The incidence of venous thromboembolism (VTE) varies widely in the literature from 7 to 100% without the use of prophylaxis. In one of the largest series to date, Jones et al12 reviewed over 16,000 SCI patients in the state of California over an 11-year period to determine thromboembolic risk factors. The authors noted that the incidence of VTE did not change with varying methods of prophylaxis. The diagnosis of DVT begins with a daily physical examination. The use of ultrasound has become widely accepted, but venography still remains the gold standard. Screening ultrasounds prior to surgical procedures can prevent perioperative morbidity. Various authors have published recommendations for VTE prophylaxis in SCI patients.13–15 Current recommendations include a combination of mechanical and pharmacologic treatments (Table 14.2). The goal with newer pharmacologic agents is to reduce the risk of complications associated with anticoagulation. Recent literature has looked at various pharmaceuticals, but no study has shown improved efficacy over traditional agents.16–18 Table 14.2 Recommendations for Deep Vein Thrombosis Prophylaxis15
 Epidemiology
Epidemiology
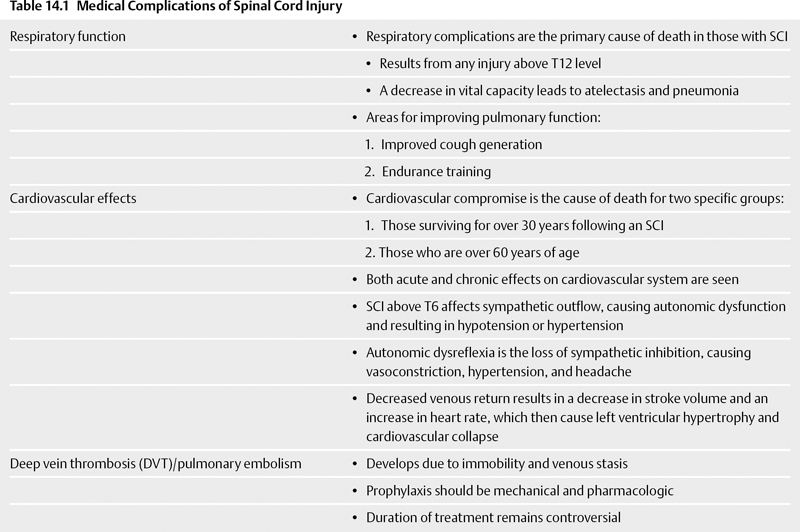
 Medical Complications (Table 14.1)
Medical Complications (Table 14.1)
Respiratory Function
Cardiovascular Effects
Deep Vein Thrombosis/Pulmonary Embolism
| • Compression hose and mechanical compression devices should be applied for the first 2 weeks following injury. |
| • In patients whose prophylaxis has been delayed by 72 hours after injury, a screening lower extremity ultrasound should be performed prior to mechanical prophylaxis. |
| • Vena cava filter placement is indicated in SCI patients who have failed anticoagulant prophylaxis or who have a contraindication to anticoagulation (active or potential bleeding sites not amenable to local control (e.g., the central nervous system, gastrointestinal tract, or lungs). |
| • Filters should also be considered in patients with complete motor paralysis due to lesions in the high cervical cord (C2, C3), with poor cardiopulmonary reserve, or with a thrombus in the inferior vena cava despite anticoagulant prophylaxis. |
| • Filter placement is not a substitute for thromboprophylaxis, which should be commenced as soon as feasible. |
| • Anticoagulant prophylaxis with either low molecular weight heparin (LMWH) or adjusted dose unfractionated heparin should be initiated within 72 hours after spinal cord injury provided there is no active bleeding or coagulopathy. |
| • Anticoagulants should be continued for 8 weeks in patients with uncomplicated complete motor injury and for 12 weeks in patients with complete motor injury and other risk factors (e.g., lower limb fractures, a history of thrombosis, cancer, heart failure, obesity, or age over 70). |
| • Reinstitution of prophylactic measures should be considered in chronic SCI patients if they are immobilized with bed rest for a prolonged period of time, are readmitted for medical illnesses, or undergo surgical procedures. |
| • Early mobilization and passive exercise should be initiated as soon as the patient is medically and surgically stable. |
| • With documented deep vein thrombosis, mobilization and exercise of the lower extremities should be delayed 48 to 72 hours until appropriate medical therapy is implemented. |
| • In symptomatic patients, perform ultrasound of the lower extremities or ventilation/perfusion lung scanning. |
| • If clinical suspicion is strong but the tests are negative or indeterminate, obtain venography of the legs, spiral computed tomography of the lungs, or pulmonary angiography. |
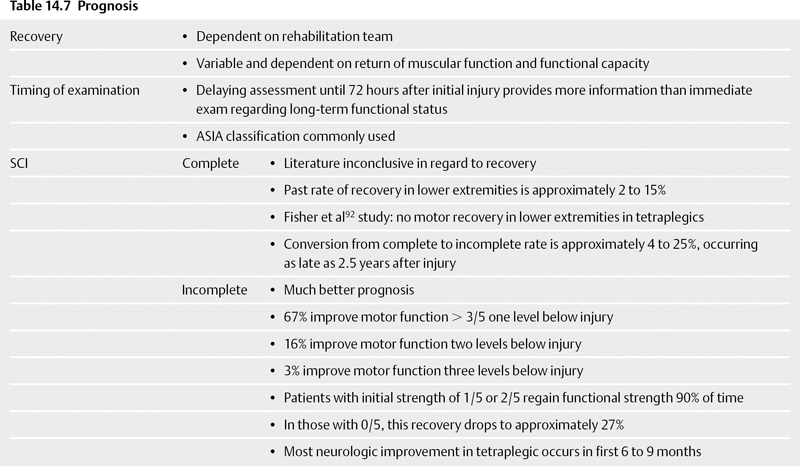
The duration of prophylaxis remains controversial. Anecdotally, many physicians continue prophylaxis until the patient regains mobility. Some physicians stop prophylaxis in the setting of spastic paralysis. Multiple studies have shown a significant decrease in the incidence of VTE after the first 3 months irrespective of the level of mobility.14 Analysis of other patient groups suggests that chronic immobility does not predict VTE.19
Heterotopic Ossification
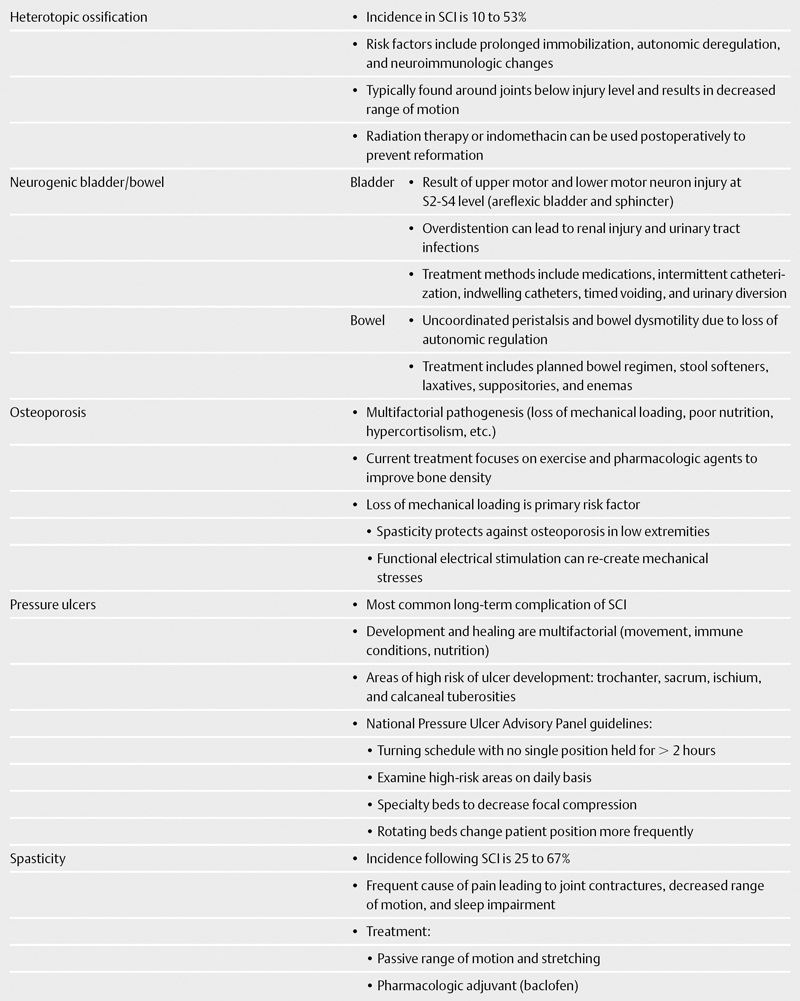
Heterotopic ossification (HO) is a known complication of SCI, with an incidence ranging from 10 to 53%.20 The exact mechanism by which HO occurs is unknown; however, risk factors include prolonged immobilization, autonomic deregulation, and neuroimmunologic changes. HO is typically observed around joints below the level of injury and usually manifests as a decreased range of motion.
Diagnosis is most easily accomplished with plain radiographs, although triple-phase bone scanning is positive earlier and is more sensitive. Serum levels of alkaline phosphatase are elevated in HO and can be used to monitor the effectiveness of treatment.
Surgical excision of HO may be considered in severe cases with the primary goal of increasing the range of motion of the affected joint. Radiation therapy or indomethacin can be used postoperatively to prevent reformation. Early passive motion is effective at reducing joint contractures and may reduce HO formation; however, excessive exercises should be avoided to prevent microtrauma-inducing HO.
Pharmacologic HO prophylaxis is the mainstay of treatment. Bisphosphonates have been shown to decrease the formation of HO, but rebound ossification after cessation of therapy has been reported.21,22 Current research is evaluating the use of nonsteroidal antiinflammatory drugs in the immediate postinjury period to prevent HO formation. In a prospective, randomized, double-blind placebocontrolled study, researchers analyzed the use of a selective cyclooxygenase-2 (COX-2) inhibitor in the prevention of HO after SCI.23 Patients who received the COX-2 inhibitor were 2.5 times less likely to develop HO. Similar results with indomethacin were also noted by the same group of authors.24
Neurogenic Bladder
Injury to upper and lower motor neurons at the S2-S4 level result in an areflexic bladder and sphincter, causing overflow incontinence. Chronic bladder overdistention causes renal injury and predisposes patients to acquiring urinary tract infections (UTIs). The goal of treatment is to prevent urinary incontinence, avoid renal injury from excessive bladder pressures, and minimize the likelihood of infection. Treatment involves medications, intermittent catheterization, indwelling catheters, timed voiding, and urinary diversion. Cholinergic agonists such as bethanecol may promote bladder contraction, whereas antimuscarinic agents (oxybutynin) are effective for treating bladder spasm.
Current research regarding neurogenic bladders after SCI is limited. Laboratory studies of dietary glycine supplementation have shown reduced bladder and urethral activity, but human studies have not been performed.25 The use of stents to treat detrusor sphincter dyssynergia is currently recommended only as a temporary option.26 Future treatment options may include the use of an artificial sphincter, and initial studies appear promising.27
Neurogenic Bowel
Spinal cord injury has two major effects on bowel function. First, loss of autonomic regulation leads to uncoordinated peristalsis and bowel dysmotility, causing both constipation and diarrhea.28 Consensus guidelines were developed in 1998 for the treatment of neurogenic bowel and are up to date as of 2005.29 Patients should be placed on a planned bowel regimen that incorporates the use of stool softeners, laxatives, suppositories, and enemas.29,30 Prokinetic agents are being added to bowel programs to help induce gastric emptying.28,31
The second effect of SCI is loss of anal sphincter control. Diagnostic testing includes anal manometry and electromyography of the external anal sphincter. Medications to increase resting tone are under investigation, as are methods for electrodefecation.31,32 Biofeedback may prove to be an effective method for improving sphincter function in partial injuries, but thus far the literature is inconsistent as to its efficacy.33
Osteoporosis
The pathogenesis of osteoporosis is multifactorial, with contributions from the lack of mechanical loading, poor nutritional status, hypercortisolism, and alterations in gonadal function.34 Osteoporosis predisposes patients to fragility fractures, particularly of the lower extremities. Current treatment regimens focus on both functional exercise and pharmacologic therapies to improve bone density.
Loss of mechanical loading is the primary risk factor for developing osteoporosis.35–42 Studies have demonstrated that spasticity protects against osteoporosis in the lower extremities, further suggesting the importance of mechanical loading.43 Previous attempts at standing the patient have met with inconsistent results.44 Functional electrical stimulation (FES) has been a novel mechanism for re-creating the mechanical stresses induced by ambulation. Chen et al45demonstrated that FES cycling temporarily was effective in increasing bone density. In stark contrast to Chen’s results, Clark et al46 found no beneficial effect of FES in improving bone mineral density.
The medical treatment of osteoporosis has become standard; however, its use to prevent SCI-induced osteoporosis is still being evaluated. Chen et al47 demonstrated that the combined use of calcitriol with pamidronate decreased bony resorption after SCI. Other studies have also shown a decrease in postinjury resorption and hypercalcemia with the use of pamidronate.48 A more recent study looked at the use of pamidronate alone to prevent osteoporosis in SCI patients.49 The authors did not find any long-term therapeutic benefit with the use of pamidronate alone. Nance et al50 found that the early use of pamidronate combined with ambulation within 6 months of injury prevented osteoporosis.
Pressure Ulcers (Table 14.3)
Pressure ulcers are the most common long-term complication following SCI.51 The development and subsequent healing of pressure ulcers are determined by multiple factors, including the frequency of patient movement, immunologic conditions, and nutritional status. Areas at high risk for the development of pressure ulcers include trochanteric, sacral, ischial, and calcaneal tuberosities.
The National Pressure Ulcer Advisory Panel (NPUAP) has developed guidelines for both the prevention and treatment of pressure ulcers.52 Patients should be put on a turning schedule, with no single position maintained for more than 2 hours to avoid tissue ischemia. High-risk areas in bed-bound patients should be examined on a daily basis. Specialty beds made of foam, air, gel, or water can be used to reduce focal compression. Newer rotating beds facilitate changes in patient position on a more frequent basis.
Treatment of pressure ulcers consists of removal of the pressure source, débridement of nonviable tissue, and establishment of a moist environment in which the tissue can heal. Modalities for débridement of necrotic tissue include surgical, mechanical, enzymatic, biologic, and ultrasound-assisted.53 Frequently, a combination of multiple modalities is required. Negative pressure therapy has been shown to be effective for large, more advanced ulcers.53
Spasticity
The incidence of spasticity after SCI ranges from 25 to 67%. 54–57 Spasticity is a frequent cause of pain in patients with SCI, leading to joint contractures, loss of range of motion, and sleep impairment. Treatment with passive range of motion and stretching can minimize muscle spasm, but pharmacologic adjuvants are usually required. Fortunately, current oral pharmacologic agents are effective at treating spasticity.
Baclofen is the first-line agent in treating spasticity after SCI. If patients do not respond to oral baclofen, medications are added, including diazepam, dantrolene, gabapentin, and tizanidine. Clinical failure of these medications has led to the use of implantable baclofen pumps. A Cochrane review of pharmacologic treatments for spasticity demonstrated that there is very limited level I evidence to support the use of these medications.58 Additional studies have been performed to assess the value of intrathecal pumps and botulinum toxin.59 These compounds are used only in cases of failed oral pharmacologic management.
 Rehabilitation (Table 14.4)
Rehabilitation (Table 14.4)
Rehabilitation of the SCI patient focuses on improving mobility, improving self-care ability, and reconditioning to improve exercise tolerance.
Self-Care
The techniques for improving self-care are well established. Areas of focus include bathing/grooming, nutritional management, medications, mobility/transfers/safety, skin/bladder/bowel management, and dressing. Patient evaluation through the Self-Care Assessment Tool (SCAT) can be used to follow the patient’s progress through therapy.60,61 Self-care focuses on the use of adaptive equipment, such as splints, bed rails, transfer boards, and wheelchairs. SCI patients work with occupational therapists to improve functionality.
Table 14.3 Staging of Pressure Ulcers93
| • Stage 1: Pressure-related alteration of intact skin whose indicators as compared with an adjacent or opposite area on the body may include the following: skin temperature (warmth or coolness), tissue consistency (firm or boggy), or sensation (pain, itching) |
| • Stage 2: Partial-thickness skin loss involving epidermis, dermis, or both. The ulcer is superficial and presents clinically as an abrasion, blister, or shallow crater. |
| • Stage 3: Full-thickness skin loss involving damage to, or necrosis of, subcutaneous tissue that may extend down to, but not through, underlying fascia. The ulcer presents clinically as a deep crater with or without undermining of the adjacent tissue. |
| • Stage 4: Full-thickness skin loss with extensive destruction, tissue necrosis, or damage to muscle, bone, or supporting structures (e.g., tendon, joint, or capsule). Undermining and sinus tracts also may be detected. |
Table 14.4 Spinal Cord Injury Rehabilitation
| Focus | Improve mobility, improve self-care ability, reconditioning |
| Self-care | • Techniques: use of adaptive equipment (splints, bed rails, transfer boards, and wheelchairs) |
| • Progress evaluated through Self-Care Assessment Tool (SCAT) | |
| • Areas of focus: bathing/grooming, nutrition, medications, mobility, dressing, skin/bowel/bladder management | |
| Exercise therapy | • Role: prevent joint contractures and improve mobility |
| • Strengthen muscles with partial or no motor injury to compensate for what is lost | |
| • Passive range of motion to maintain joint mobility | |
| • Electrical stimulation and complex devices (Parastep system) show promise | |
| • Autonomic dysreflexia can be induced by exercise; treat immediately: | |
| • Position patient upright to decrease intracranial pressure | |
| • Administer vasodilators | |
| • Avoid beta-blockers | |
| • Strength conditioning limited to prevent fracture | |
| • Exercise can also induce hyperthermia due to thermal dysregulation | |
| Exercise tolerance | • Programs tailored after assessing cardiac and pulmonary status |
| • Combine strength with endurance training to improve cardiovascular function | |
| • Combine electrical stimulation with endurance training to prevent atrophy and improve glucose tolerance |
Exercise Therapy
The role of exercise therapy is to prevent joint contractures and to improve patient mobility. Exercise is used to strengthen muscles with partial or no motor injury to better compensate for denervated muscles. Passive range-of-motion exercises maintain joint mobility for future function. Electrical stimulation of nonfunctional muscle groups has not been shown to have an effect on long-term function.62 Complex devices such as the Parastep system have been developed incorporating functional electrical stimulation to promote ambulation.63
Autonomic dysreflexia can be induced by exercise or noxious stimuli. Practitioners should recognize this complication during exercise therapy and treat it immediately. Treatment includes positioning the patient upright to decrease intracranial pressure, administering vasodilators, and avoiding the use of beta-blockers.
Care must also be taken to avoid fractures in patients with SCI. Generalized osteopenia combined with spasticity puts patients at high risk for fracture. Strength conditioning should be limited to prevent this complication.
Spinal cord injury patients also have thermal dysregulation and can develop hyperthermia with excessive sweating.11 Exercise therapies should be performed in cool areas, and the importance of rehydration should be emphasized.
Exercise Tolerance
Reconditioning the deconditioned SCI patient is challenging. Specific exercise therapies are similar to conditioning regimens used in normal individuals. Programs are tailored after assessing cardiovascular and pulmonary status. Strengthening exercises are combined with endurance training to improve cardiovascular status. Current research evaluates the use of electrical stimulation combined with endurance training to prevent muscular atrophy and improve glucose tolerance.64–68
 Psychosocial Issues (Table 14.5)
Psychosocial Issues (Table 14.5)
Spinal cord injury not only affects patients physically but also affects their mental health and social interactions. Patients with SCI are more likely to suffer from depression. Rates of depression in SCI-affected patients range from 14 to 35%, with severe symptoms seen in 15% of patients.69 Patients with depression are more likely to have decubitus ulcers and UTIs.70 Factors that have been associated with an increased risk of depression include greater severity of injury, less social support, lower level of education, history of substance abuse, and lower functional ability.71 Suicide is one of the top preventable causes of death after SCI, and it accounts for up to 7% of mortality after SCI injury, with gunshot wounds, drowning, and overdose.72
Table 14.5 Psychosocial Issues of Spinal Cord Injury
| Depression | • Rates of approximately 14 to 35% in SCI patients |
| • More likely to develop decubitus ulcers and urinary tract infections (UTI) | |
| • Factors associated with increased risk of depression: greater severity of injury, less social support, lower level of education, history of substance abuse, lower functional ability | |
| Suicide | • 7% of mortality after SCI injury |
| • Most common: gunshot wounds, drowning, overdose | |
| Return to work | • Rates increase with increased survival following SCI |
| • 13 to 69% rate of employment | |
| • Education level prior to injury is predictive factor |
As more people survive SCI, the rate of return to the work force is increasing. Rates of employment from previous studies vary from 13 to 69%, depending on location and the definition of employment.73–76 Educational level before injury is one of the most predictive factors of returning to work after SCI.77–80 People with previously lighter types of work are also more likely to return to work after SCI.81
Sexual Dysfunction (Table 14.6)
Sexual dysfunction after SCI involves neurologic, physical, and psychosocial issues. Two pathways for erection include the reflex pathway, which is based on cutaneous stimulation, and psychogenic pathways that incorporate mental imagery, previous experiences, and senses of the patient. Parasympathetic innervation from the S2-S4 levels controls arteriovenous shunts in the corpora cavernosa to induce erection. The sympathetic centers responsible for ejaculation are located at the T11-L2 levels. Pharmacologic agents including sildenafil are effective at treating erectile dysfunction, allowing for functional erection in up to 80% of SCI patients.82,83 Assistive devices such as rings, vacuum devices, and artificial penile prostheses can be used in addition to pharmacotherapy.
Electroejaculation and vibratory penile stimulation (VPS) have been used to assist in ejaculation. Electroejaculation works by placement of an electrode on the rectal wall near the prostate to stimulate contractions for ejaculation. VPS activates the ejaculatory reflex by vibratory stimulation of the frenulum.
In women, psychogenic and reflex stimulation influences vaginal lubrication. Women with lesions between C4 and C8 are able to have orgasms; however, the frequency is diminished, and the time to orgasm is prolonged. Peer counseling has also been shown to be beneficial for women for sexual function.73 Finally, helping patients with other SCI-associated complications such as spasticity, bowel and bladder incontinence, and autonomic dysfunction will likely improve the self-esteem of both men and women, thereby improving sexual function and orgasm.
Table 14.6 Sexual Function Following Spinal Cord Injury
| Pathways for erection | 1. Reflex pathway: based on cutaneous stimulation |
| 2. Psychogenic pathway: mental imagery, previous experience, and senses | |
| Parasympathetic innervation | S2-S4 levels; induces erection |
| Sympathetic innervation | T11-L2 levels; responsible for ejaculation |
| Pharmacologic agents | Sildenafil: functional erection in approximately 80% of SCI patients |
| Assistive devices | • Rings, vacuum devices, and artificial penile prostheses can be used |
| • Electroejaculation and vibratory penile stimulation used to assist ejaculation | |
| Women | • Lesion site between C4 and C8: can still have orgasms; frequency decreases, and time to orgasm is prolonged |
| • Peer counseling and improving self-esteem are beneficial |
< div class='tao-gold-member'>