CHAPTER 16 Recurrent Ischemia after Reperfusion Therapy for Acute Myocardial Infarction
RECURRENT ISCHEMIA after acute myocardial infarction (MI) is a heterogeneous process. It is most often caused by reocclusion of the infarct-affected artery by thrombosis, but also may occur as a result of spasm, extension of subintimal hemorrhage (usually after angioplasty at that site), inadequate collaterals to the same territory in the face of increased myocardial oxygen demand, or “ischemia at a distance”1 unrelated to the initial culprit site. Recurrent ischemia also may result in processes as disparate as recurrent MI or occult reocclusion without any symptoms.
Predictors
Symptomatic recurrent ischemia after presentation with MI is more likely in patients treated with thrombolysis rather than primary percutaneous coronary intervention (PCI) (see later), patients with recurrent electrocardiogram (ECG) changes, patients with substantial myocardial salvage, patients with high-grade residual stenoses, and patients with augmented platelet and thrombin activation. Demographic and clinical variables have not proved to be helpful in predicting which patients will develop reocclusion.2,3 One large series found, however, that clinical predictors of reinfarction included advanced age, shorter time to thrombolysis, nonsmoking status, prior MI or angina, female sex, anterior infarction, and lower systolic blood pressure.4 Other authors have reported that right coronary artery infarcts and infarcts associated with bradycardia on admission are more prone to reocclusion.5
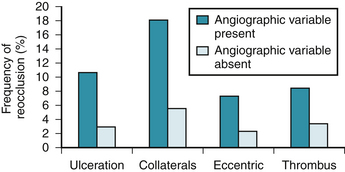
The TIMI-4 trial attempted to identify angiographic predictors of reocclusion after thrombolysis.6 TIMI grade 2 flow, lesion ulceration, presence of collaterals, and eccentric lesions were associated with a significantly higher rate of infarct-related artery reocclusion (Fig. 16-1). In contrast to these findings, other studies, such as GUSTO and TAMI, did not find angiographic features at a 90-minute baseline study to be helpful for predicting reocclusion.7,8 There were, however, important differences among these trials that could explain the different findings.6 Other authors have associated the following characteristics with persistent symptoms, adverse in-hospital outcomes, or reocclusion: lesion ulceration,9–12 high-grade residual stenosis and reduced lumen area,13–17 and lack of restoration of normal coronary flow (TIMI flow <3).18–22
Incidence
In the era before reperfusion, recurrent angina with or without ECG changes occurred in 19% to 37% of patients, but reinfarction was far less common. Recurrent ischemia was more frequently seen in patients who had not initially developed a Q wave MI,23–26 although this classic teaching has not been a uniform finding.26 After coronary reperfusion, within the first few hours of infarction, the risk of recurrent ischemia is higher than without reperfusion, presumably because the risk is higher with an open infarcted artery and salvaged myocardium. In a meta-analysis of nearly 50,000 patients from six large, placebo-controlled thrombolytic trials of patients usually treated less than 6 hours from the onset of symptoms, reinfarction occurred in 3.6% of patients treated with thrombolytic agents compared with 3% of patients who did not undergo thrombolysis (P < .001).27–32 In the two placebo-controlled thrombolytic trials in which all patients had symptoms for at least 6 hours before treatment (i.e., EMERAS33 and LATE34), there was no difference in the incidence of reinfarction between patients treated with or without thrombolysis (2.4% versus 2.9%; P = .10), presumably because MIs treated later had less salvaged myocardium.
After apparently successful fibrinolysis by clinical criteria, and despite adjunctive pharmacologic therapy, early recurrence of ischemia or ST segment shifts (threatened reocclusion) have been observed in 20% to 30% of patients,35,36 thrombotic coronary reocclusion has been observed in 5% to 15% before hospital discharge and in 25% by 3 months,2,3,37–39 and early reinfarction has been observed in 3% to 5%.4,39–43 When symptomatic reocclusion occurs after thrombolytic therapy, it generally occurs (80% of the time) within the first 2 days after treatment. Only a few reocclusions occur during the initial hospitalization, however. Reocclusion is clinically silent in more than 50% of cases and tends to occur later.3 In the APRICOT study,38 300 patients treated with recombinant tissue plasminogen activator (rt-PA) with patent infarct-affected arteries, documented by angiography 24 to 48 hours after treatment, underwent protocol-required repeat cardiac catheterization 3 months later. The patients were further randomly assigned to treatment with aspirin, warfarin, or neither drug. At follow-up, 25%, 30%, and 32% in the three groups had reocclusions, only 25% of which were associated with a recurrent MI. Because reocclusion can be clinically silent, late angiography remains the gold standard in assessing the frequency of reocclusion.
In an overview by Granger and colleagues44 of 1183 patients with paired angiograms, 14% of patients receiving rt-PA versus 8% of patients treated with non–fibrin-specific agents had reocclusion (P = .002). This finding was not confirmed, however, in the similarly sized angiographic arm of the GUSTO-I study, possibly because of the more vigorous treatment of patients receiving rt-PA with heparin in the latter study. Also, patients treated with accelerated or weight-adjusted rt-PA regimens consistently had lower rates of reocclusion than patients treated with the original U.S. Food and Drug Administration–approved 3-hour dosing regimen.5,45 In two reviews of almost 76,000 patients from GUSTO-I, GUSTO-III, TIMI, and InTIME II trials, reinfarction occurred in 4.3% of patients at a median of 2 to 4 days after fibrinolytic therapy, and was independent of the fibrinolytic agent used.4,39
Reinfarction might occur in a new territory, rather than reflecting failed fibrinolysis in the index territory. This issue was addressed in a report from the HERO-2 trial of bivalirudin versus unfractionated heparin before streptokinase administration. Confirmed reinfarction occurred in 552 patients (3.2%). Among these patients, 4% (0.15% of all patients) had ST segment elevation in a new territory at a mean of 46 hours.46
Multiple randomized trials have been done to compare PCI with thrombolytic therapy. PCI enhances survival with a lower rate of intracranial hemorrhage and recurrent MI. In a review of 23 trials, primary PCI was better than thrombolytic therapy at reducing death (7% versus 9%; P = .0002) and nonfatal reinfarction (3% versus 7%; P < .0001). The review included 7739 patients; streptokinase was used in 8 trials (n = 1837), and fibrin-specific agents were used in 15 trials (n = 5902). Stents were used in 12 trials, and platelet glycoprotein (GP) IIb/IIIa inhibitors were used in 8 trials. The results seen with primary PCI remained better than the results seen with thrombolytic therapy during long-term follow-up, and were independent of the type of thrombolytic agent used, and whether or not the patient was transferred for primary PCI.47
Several randomized trials (DANAMI-2, STAT, STOPAMI-1, and STOPAMI-2) have shown a better outcome with primary stenting compared with thrombolysis.48–51 The largest is the DANAMI-2 trial, which was discontinued prematurely because of a significant reduction in the primary end point of mortality, reinfarction, or stroke at 30 days with PCI (8% versus 13.7%). The benefit was primarily due to less reinfarction.48
The clinical efficacy of primary balloon angioplasty in acute ST segment elevation myocardial infarction (STEMI) and other settings is limited by the risks of early reocclusion and late restenosis, providing the rationale for the use of intracoronary stents. Initial nonrandomized studies suggesting that primary stenting was more effective than primary balloon angioplasty52–54 were followed by randomized trials.55–58 A 2005 meta-analysis evaluated nine randomized trials comprising 4433 patients.59 Stenting was associated with significant reductions in reinfarction (odds ratio [OR] 0.52 at 30 days, OR 0.67 at 1 year) and target vessel revascularization (OR 0.45 at 30 days, OR 0.47 at 1 year). There was no significant difference in mortality (OR 1.06 at 1 year). Finally, in a 2007 meta-analysis of eight randomized trials of drug-eluting stents versus bare metal stents involving 2786 patients, reinfarction was less common with drug-eluting stents (OR 0.72), whereas there was no difference in stent thrombosis (OR 0.86) or mortality (OR 0.76).60
Consequences
The consequences of reocclusion depend partly on the amount of newly ischemic myocardium, the capacity of the remaining myocardium to compensate, and whether adequate coronary flow is promptly re-established. Whether symptomatic or silent, reocclusion is associated with significant morbidity and mortality in the short-term and the long-term. In the report by Ohman and coworkers3 from the TAMI study, reocclusion was associated with about a threefold risk of worsened ventricular function, heart failure, and conduction abnormalities, and a higher in-hospital mortality (P = .01) than maintained coronary patency (Table 16-1).
Table 16–1 Consequences of Coronary Reocclusion after Thrombolytic Therapy for Acute Myocardial Infarction∗
| Clinical Event | Infarct Artery Patent (% of Patients) | Infarct Artery Reoccluded (% of Patients) |
|---|---|---|
| Improvement in LVEF, baseline to day 7 | +3 | −1 |
| Pulmonary edema | 4 | 19 |
| Sustained hypotension | 17 | 25 |
| Second-degree or third-degree AV block | 13 | 25 |
| In-hospital death | 5 | 11 |
AV, atrioventricular; LVEF, left ventricular ejection fraction.
∗ TAMI trial (N = 810 patients).
In the University of Michigan 1984-1990 experience,61 of 405 patients with paired baseline and prehospital discharge angiography, the mortality rate for patients with sustained reperfusion was 2%, but it was 15% after reperfusion and reocclusion. Risk factors for death after recurrent ischemia were advanced age, hemodynamic instability at the time of initial presentation, multivessel disease, and failure to obtain successful reperfusion within 90 minutes of recurrent ischemia (all P < .05).61
In two reviews of almost 76,000 patients from GUSTO-I, GUSTO-III, TIMI, and InTIME II,4,39 patients with reinfarction had a higher overall mortality rate at 30 days (11.3% to 16.4% versus 3.5% to 6.2% without reinfarction), which could be markedly reduced with PCI during index hospitalization.4,39 The data were conflicting as to whether 30-day survivors of reinfarction do4,39 or do not4,39 have a modest increase in risk from 30 days to 1 year. In the TIMI and InTIME II trials, most deaths occurred early, and the risk of additional deaths between the index hospital period and 2 years was not significantly increased in patients with recurrent MI.39
Prevention
The thrombus associated with reocclusion after thrombolytic therapy or stenting is typically platelet-rich62 and resistant to further thrombolysis. The effect of thrombolytic agents on platelet function is complex, involving thrombin activation and perturbation of the GP IIb/IIIa and the GP Ib receptors, but in aggregate, platelet activation is often demonstrable.63,64
The effect of aspirin, a weak platelet antagonist, has been studied in several trials, most notably ISIS-2.29,65 Reinfarction was essentially halved with aspirin usage in ISIS-2 (1.8% versus 3.3%; P < .0001), and an overview suggested that reocclusion was similarly reduced (11% versus 25%; P < .001).
Thienopyridines were initially considered as an alternative to aspirin in patients with STEMI. The benefit of this approach was suggested by results from the CAPRIE trial, which was not limited to STEMI, and the STAMI trial, which showed that clopidogrel and ticlopidine are at least as effective as aspirin.66–68 The role of clopidogrel in addition to aspirin must be considered in the context of the form of reperfusion therapy (primary PCI, thrombolysis, or no reperfusion). In the setting of STEMI treated with thrombolytic therapy, two randomized trials (CLARITY-TIMI 28 and COMMIT/CCS-2) showed clear evidence of benefit from early administration of clopidogrel in addition to aspirin.69,70
No randomized trials have specifically evaluated the efficacy of clopidogrel in addition to aspirin in patients with STEMI who are not reperfused. Half of patients in COMMIT/CCS-2, which showed benefit from clopidogrel therapy, were not reperfused, however.69 Most physicians also extrapolate from the benefit seen with clopidogrel in patients with non–ST segment elevation acute coronary syndromes who were not revascularized in the CURE trial.71 No randomized trials have evaluated the efficacy of clopidogrel in addition to aspirin in patients with STEMI who are treated with primary PCI. Most clinicians give clopidogrel based on extrapolation from randomized trials (PCI-CURE and CREDO) in patients with non–ST segment elevation acute coronary syndromes. These trials showed that clopidogrel improves short-term and long-term cardiovascular outcomes—reduction of acute thrombotic complications—when given in addition to aspirin in patients treated with PCI.72,73
Along with aspirin, heparin has also been a standard treatment after thrombolytic therapy. GUSTO-I results suggest that heparin is unnecessary when streptokinase is the thrombolytic agent used, but several studies have suggested that heparin improves short-term patency after rt-PA use.74,75 Hsia and colleagues76 observed that the degree of elevation of activated partial thromboplastin time (aPTT), not the use of heparin, was crucial in determining patency after rt-PA use. In the HART study, if the aPTT in the first 12 hours after rt-PA use was less than 45 seconds, only 45% of infarct-affected arteries were patent at follow-up, but if the aPTT was longer than 60 seconds, 95% of vessels were open. Higher aPTT values are associated with a greater risk of bleeding. Data from the much larger GUSTO-I study suggest that the optimal compromise in reducing both risks is to have the aPTT between 63 and 72 seconds.77
Two randomized studies addressed the issue of how long heparin should be continued in this setting.78,79 Both studies concluded that heparin could be safely discontinued in most patients after 24 hours. American College of Cardiology (ACC)/American Heart Association (AHA) 2004 STEMI guidelines recommended infusions of heparin routinely for 48 hours, and for a longer period only in patients with an ongoing indication for anticoagulation.80–82
Although no new trials specifically focusing on heparin in STEMI have been reported, numerous studies have compared alternative anticoagulant regimens (reviparin, fondaparinux, or enoxaparin) with heparin or placebo. In these studies, the new regimen was administered for the duration of the index hospitalization. The CREATE (reviparin versus placebo),83 OASIS-6 (fondaparinux versus placebo or heparin),84 and ExTRACT TIMI-25 (enoxaparin versus heparin)85 trials provided a rationale for a new recommendation of anticoagulation therapy for a minimum of 48 hours, preferably extended to the duration of the index hospitalization (up to 8 days) for patients undergoing reperfusion with fibrinolytics.86 In the reperfused cohort of the CREATE trial, reviparin was associated with fewer deaths, reinfarctions, and strokes (11% versus 12.3% at 30 days). Reviparin was associated, however, with higher rates of life-threatening bleeding (1.1% versus 0.4% at 30 days).83,86 In the subset of OASIS-6 trial patients submitted to thrombolysis, fondaparinux was superior to control therapy (placebo or unfractionated heparin) with lower rates of death, reinfarction and severe hemorrhage at 30 days.86,89 This benefit was not observed in the cohort submitted to primary PCI, with a trend toward worse outcome.84,86
In the ExTRACT-TIMI 25 trial, enoxaparin compared with unfractionated heparin was associated with lower rates of death and reinfarction (9.9% versus 12% at 30 days), but with higher rates of severe hemorrhage (2.1% versus 1.4% at 30 days). Findings were consistent among subgroups, regardless of thrombolytic agent type, age (dose was adjusted for patients >75 years old), or postlysis PCI.85,86 In the 2007 update ACC/AHA STEMI guidelines, unfractionated heparin, enoxaparin, and fondaparinux were considered as anticoagulation regimens with established efficacy as ancillary therapy to thrombolysis.86 Anticoagulation regimens with unfractionated heparin or low-molecular-weight heparins should be maintained if patients proceed to PCI, without crossover to other agents.86 Fondaparinux is associated, however, with catheter thrombosis, and additional anticoagulation with anti–factor IIa activity, such as unfractionated heparin or bivalirudin, is recommended.86
The search for better agents led to direct thrombin inhibitors and to antagonists of the final common pathway of platelet aggregation, the GP IIb/IIIa receptor. Direct thrombin inhibitors have the putative advantages over heparin of accessing clot-bound thrombin, having no reliance on antithrombin III (in which some patients are deficient), having no natural inhibitors, and being easier to regulate therapeutic levels.88 The phase II TIMI-5 study suggested that these characteristics would translate into clinical benefits, with greater coronary patency after rt-PA use with hirudin than heparin (98% versus 89%; P = .01) and with a reduction in in-hospital death or reinfarction (7% versus 17%; P = .02).89 These results were not confirmed by the larger GUSTO-IIa and TIMI-9a studies,87,90 however, both of which were stopped prematurely because of an excess of bleeding and strokes with hirudin.
Clinical experience with bivalirudin in the setting of acute MI has shown some benefit.91,92 In a pilot trial (HERO) of 412 patients receiving streptokinase and aspirin, a dose-related improvement in TIMI grade 3 flow was observed with bivalirudin in 48% of patients in the high-dose bivalirudin group, 46% of patients in the low-dose bivalirudin group, and 35% of patients in the heparin group. There were no differences in reocclusion or clinical end point event rates, and major bleeding seemed to be less frequent with bivalirudin. Based on these favorable findings, bivalirudin was compared with unfractionated heparin in the HERO-2 mortality trial in 17,073 patients receiving streptokinase.91 At 30 days, there was no difference in mortality rates (10.5% versus 10.9% with heparin); there was a small but significant reduction in reinfarction at 96 hours with bivalirudin (1.6% versus 2.3%); there was a nonsignificant trend toward more episodes of severe bleeding (0.7% versus 0.5%; P = .07) and intracerebral bleeding (0.6% versus 0.4%; P = .09) with bivalirudin; and there was a significant increase in moderate and mild bleeding with bivalirudin.
In the HORIZON AMI trial, patients treated with primary PCI (stenting) were randomly assigned to unfractionated heparin plus a GP IIb/IIIa inhibitor (abciximab or eptifibatide), or to bivalirudin monotherapy stopped at the end of the procedure plus provisional GP IIb/IIIa inhibitors for large thrombus or refractory no-flow. Only 7.2% of patients in the bivalirudin-treated group also received GP IIb/IIIa inhibitors in the catheterization laboratory. At 30 days, investigators saw a 24% reduction in net adverse clinical events and a 40% reduction in major bleeding—the primary end points of the study. Major adverse cardiac events, defined as all-cause death, reinfarction, ischemic target vessel revascularization, or stroke, were not different between the two groups. There were no significant differences between any of the individual event rates that made up the major adverse cardiac events end point, with the exception of cardiac mortality, which was significantly reduced in patients in the bivalirudin arm of the study.93
Combination therapy using a half-dose of a thrombolytic agent with a GP IIb/IIIa inhibitor may be more likely to restore coronary perfusion; promote ST segment resolution at 90 minutes; and reduce the incidence of recurrent ischemia, nonfatal reinfarction, and rescue PCI. Despite these benefits, two large trials, GUSTO V94,95 and ASSENT-3,96,97
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree