INTRODUCTION AND EPIDEMIOLOGY
Blunt thoracic injuries account for up to one fourth of all injury-related deaths.1 The mechanism of injury and severity of tissue damage predict the clinical course and outcome.2 Injuries that do not violate the pleura usually can be managed with conservative measures, such as wound management or observation. Penetrating injuries that violate the pleura typically result in pneumothorax, with an accompanying hemothorax in most cases. Treatment is generally supportive care after tube thoracostomy.
Blunt trauma produces damage by direct injury, compression, and forces of acceleration or deceleration. Patients with significant blunt injury may require intubation and mechanical ventilation and invasive procedures such as tube thoracostomy. In general, victims of penetrating injuries who survive to reach the hospital often have better outcomes than those who have sustained blunt injuries. Blunt chest trauma from blast injuries is discussed in chapter 7, “Bomb, Blast, and Crush Injuries.” Penetrating chest injuries in the “cardiac box” (see Figure 262-1), an area bounded by the sternal notch, xiphoid process, and nipples, should be presumed cardiac or great vessel injuries until proven otherwise.
CLINICAL FEATURES
The most frequent symptoms of thoracic trauma are chest pain and shortness of breath. The pain is most often localized to the involved area of the chest wall, but sometimes it is referred to the abdomen, neck, shoulder, back, or arms. Dyspnea and tachypnea are nonspecific findings and may also be caused by blood loss or pain from other injuries or by anxiety.
Rapidly perform the physical examination during the primary and secondary surveys to detect life-threatening injuries.
Inspect the chest wall for contusions, abrasions, and other signs of trauma, including a “seat belt sign” that can indicate deceleration or vascular injury. Examine the chest for signs of paradoxical segments or flail chest, intrathoracic bleeding, and open chest wounds. The patient must be making a reasonable ventilatory effort to demonstrate these injuries.
Distended neck veins may indicate the presence of pericardial tamponade, tension pneumothorax, cardiac failure, or air embolism; however, in the setting of hypovolemia, this sign may be absent. If the face and neck are cyanotic and/or swollen, then suspect severe injury to the superior mediastinum with occlusion or compression of the superior vena cava. Subcutaneous emphysema from a torn bronchus or laceration of the lung can also cause severe swelling of the neck and face.
A scaphoid abdomen may indicate a diaphragmatic injury with herniation of abdominal contents into the chest. Excessive abdominal movement during breathing may indicate chest wall damage that might not otherwise be apparent.
Breath sounds are most readily heard in the axillae. Unilaterally decreased breath sounds may indicate the presence of hemothorax or pneumothorax. If the patient has an endotracheal tube in place, assess the depth of the tube for mainstem bronchus intubation (the usual depth is no more than three times the inner diameter of the endotracheal tube—23 cm in adult males and 21 cm in adult females) before performing tube thoracostomy in these patients. Persistent decreased breath sounds on one side may also be due to a bronchial foreign body or ruptured bronchus. The presence of bowel sounds in the thorax usually indicates a diaphragmatic injury.
Palpate the neck to determine whether the trachea is midline or displaced. Palpation of the chest wall may reveal areas of localized tenderness or crepitus due to fractured ribs or subcutaneous emphysema. Localized and consistent tenderness over ribs should be attributed to rib fractures, even in the absence of findings on conventional chest radiography. Severe localized tenderness, crepitus, or a mobile segment of the sternum may be the only objective evidence of a sternal fracture. Palpation of the chest with the patient coughing or straining may detect abnormal motion of an unsTable portion of the chest wall better than visual inspection.
Sensitivity of physical examination findings is not high enough to rule in or out common thoracic injuries. A study of hemodynamically sTable chest trauma patients showed that the sensitivity of auscultation for the detection of a hemopneumothorax is only 50%.3 Consider patient symptoms, vital signs, and physical examination findings together to detect significant injuries in sTable chest trauma patients and to guide further investigation with imaging.
IMAGING
Plain chest radiographs are helpful to screen for abnormal mediastinal contours, hemothorax, pneumothorax, pulmonary contusions, diaphragmatic injury, and osseous trauma. Mark penetrating surface wounds before imaging. Most chest radiographs are initially taken in the supine position at the bedside due to concern for occult spinal cord injuries and to facilitate resuscitation. Chest radiographs frequently underestimate the severity and extent of chest trauma and may fail to detect an injury. Up to 50% of blunt chest trauma patients with normal initial chest radiography have multiple injuries on CT; however, some of these occult injuries do not change management or outcome.4,5,6,7,8 Chest x-ray is obtained to identify findings such as pneumothorax, hemothorax, aortic or great vessel injury, multiple rib fractures, sternal fracture, diaphragmatic rupture, and pulmonary contusions or lacerations. The National Emergency X-Radiography Utilization Study (NEXUS) Chest Rules were developed in a manner similar to NEXUS Cervical Spine Imaging rules to identify patients with blunt chest trauma with very low risk of thoracic injury. The rules were developed and validated in a convenience sample of 9905 patients >14 years old. If all of the NEXUS Chest criteria were absent, chest imaging (plain radiography or CT) could be omitted (Table 261-1). Patients in this cohort were not evaluated with bedside US, and decision for plain chest radiography or chest CT was left to the discretion of the caring physician. Sensitivity and specificity were 98.8% and 13.3%, respectively, for thoracic injury. Only about half of the study group underwent CT, limiting any conclusions about plain radiography versus CT in patients with blunt chest trauma.
| Blunt trauma in patients >14 y old in whom chest imaging is considered to exclude intrathoracic injury | Presence of 1 or more criteria: cannot exclude intrathoracic injury and obtain chest imaging Age >60 y old Rapid deceleration: fall >20 ft (>6 m); motor vehicle crash >40 mph (>64 km/h) Chest pain Intoxication Abnormal alertness or abnormal mental status Distracting painful Injury Tenderness to chest wall palpation |
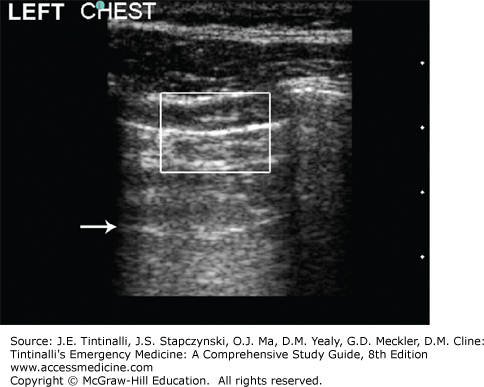
Bedside US can quickly diagnose pneumothorax, hemothorax, and pericardial tamponade as part of the FAST examination. In trained hands, US has a greater sensitivity and equal specificity for detecting hemothorax in patients with chest trauma compared with chest radiography.9 Likewise, the sensitivity of US for detecting pneumothorax approaches 92%, with near 100% specificity (as compared with 50% to 80% sensitivity and 90% specificity for chest radiographs)10; thus, US appears to have greater “diagnostic performance” than clinical exam and chest radiograph together.11 US in the ED can detect occult pneumothorax as accurately as CT.12 In addition, US has also been found helpful for describing small, medium, or large pneumothoraces with good agreement with CT (Figure 261-1).13
FIGURE 261-1.
Pneumothorax (7.5–10 MHz). Power Doppler is activated and the gain adjusted correctly by comparison with the normal right hemithorax. The patient’s left hemithorax showed a negative pleural sliding sign, no comet tail artifacts, and no power Doppler signal at the pleural interface. The specificity for pneumothorax is improved when an A-line is also visible (arrow). [Reproduced with permission from Ma OJ, Mateer JR, Reardon RF, Joing S (eds): Emergency Ultrasound, 3rd ed. McGraw-Hill, Inc., 2014. Fig 5-26, p. 82.]
CT can detect major and occult injuries and identify the need for additional interventions, but may demonstrate incidental findings that require follow-up and do not change acute care.8 CT is more sensitive for detecting pulmonary contusion and hemothorax than plain radiography.4 For penetrating lung injury, if the clinical condition allows, CT is performed to identify the extent of injury and involvement of the heart or great vessels.
OTHER DIAGNOSTIC STUDIES
Thoracic trauma often requires a contrast esophagogram to diagnose esophageal injury. Water-soluble contrast is preferred over barium-containing contrast in patients with high suspicion for esophageal rupture. Barium swallow imaging has fewer false positives, but may cause mediastinitis if there is leakage of contrast out of the esophagus.
In selected cases, such as in penetrating wounds of the chest or lower neck, bronchoscopy or esophagoscopy may be indicated to exclude an injury to the aerodigestive tract. Such studies may be deferred until the patient is resuscitated and hemodynamically stable.
GENERAL TREATMENT
Perform initial resuscitation and airway management according to established principles (see chapter 254, “Trauma in Adults”). If the patient is making little or no respiratory effort, consider CNS dysfunction due to head trauma, intoxication, or spinal cord injury. In patients with respiratory effort but with little or no air movement, suspect upper airway obstruction. Absent or abnormal breath sounds may indicate flail chest, hemopneumothorax, diaphragmatic injury, or parenchymal lung damage. Although each of these has unique therapies, respiratory distress that is not immediately relieved by specific intervention should prompt intubation and ventilation. Suspect, diagnose, and treat specific life-threatening pulmonary injuries during the primary survey. These include tension pneumothorax, massive hemothorax, and open pneumothorax.
The management of chest trauma patients with respiratory instability is challenging. Hypoxia and hypoventilation are two prevenTable causes of mortality; therefore, maintaining adequate oxygenation and ventilation in the acute chest trauma patient is essential. Monitor all trauma patients by continuous noninvasive pulse oximetry to assure adequate oxygen saturation. In patients with severe chest trauma or respiratory compromise, an arterial blood gas is helpful to monitor metabolic status and adequate oxygenation and ventilation. Metabolic acidosis with insufficient respiratory compensation is an indication for ventilatory support. Ventilatory support is indicated in chest trauma patients who continue to exhibit impaired ventilation despite measures to relieve chest wall pain and evacuate hemopneumothorax (Table 261-2).
Altered mental status Hypovolemic shock Multiple injuries Multiple blood transfusions Elderly patient Preexisting pulmonary disease Respiratory rate >30–35 breaths/min Vital capacity <10–15 mL/kg Negative inspiratory force <25–30 cm H2O |
Decompensation leading to cardiac arrest may occur during or after endotracheal intubation for reasons related to the initial injury or the procedure (Table 261-3). If the patient has poor venous return due to hypovolemia, hyperventilation may increase intrathoracic pressure and decrease venous return to the heart. In the hypovolemic patient, the resultant reduction in cardiac output can lead to cardiac arrest. Ventilate hypovolemic patients with tidal volumes of no more than 5 to 8 mL/kg and at rates not exceeding 10 to 14 times per minute. Excessive hyperventilation can also cause severe alkalosis, which shifts the oxyhemoglobin saturation curve to the left, impairing oxygen unloading at the tissue level. In the presence of pulmonary injury or preexisting bullous disease, vigorous positive-pressure ventilation can lead to tension pneumothorax, further reducing venous return.
Inadequate preoxygenation Esophageal intubation Intubation of the right or left mainstem bronchus Tension pneumothorax Systemic air embolism Decreased venous return due to excessive ventilatory rate or pressures Vasovagal response |
Unexplained bradycardia associated with assisted ventilation should prompt immediate verification of proper endotracheal tube position and exclusion of esophageal tube placement, as well as evaluation for tension pneumothorax.
LIFE-THREATENING PULMONARY INJURIES
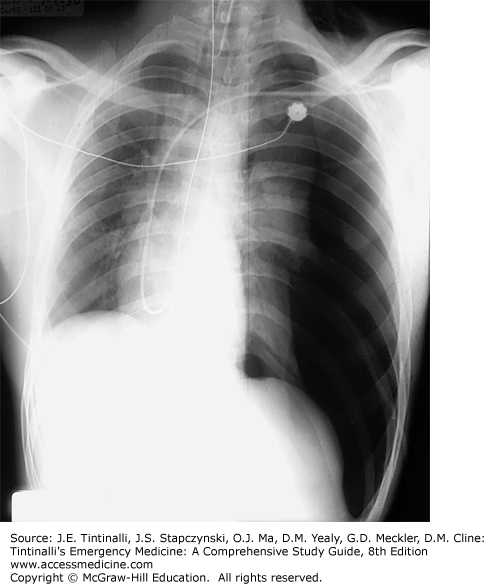
Diagnose tension pneumothorax clinically, before the chest x-ray is obtained. Although the classic presentation includes distended neck veins, hypotension or evidence of hypoperfusion, diminished or absent breath sounds on the affected side, and tracheal deviation to the contralateral side (Figure 261-2), one or more of these elements may be absent in the presence of hypovolemia. Perform immediate needle decompression.
FIGURE 261-2.
Tension pneumothorax. Supine chest radiograph of a tension pneumothorax. Note the deviation of the trachea and shifting of the mediastinal contents to the right. Tension pneumothorax should normally be diagnosed prior to chest radiograph. [Reproduced with permission from Schwartz DT (ed): Emergency Radiology, Case Studies. © 2008 McGraw-Hill Inc., Fig I-9-10.]
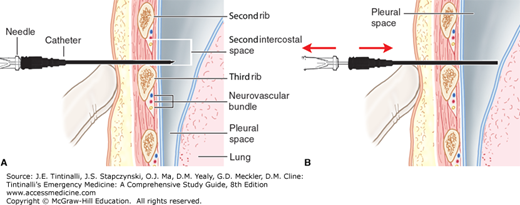
The most common approach to needle decompression is to introduce a 14-gauge IV needle and catheter into the pleural space in the midclavicular line just above the rib at the second intercostal space (Figure 261-3). An anterior midclavicular approach is important because this is the shortest distance from the skin to the pleura, avoids the internal mammary vessels that are located approximately 3 cm lateral to the sternal border, and avoids mediastinal vessels.14,15,16 A standard needle and catheter may not be long enough for decompression, as the mean chest wall thickness in the United States is 4.5 cm.15 An alternative site is the fourth to fifth intercostal space at the anterior axillary line.16 A rush of air exiting the pleural space may be audible and is diagnostic of a pneumothorax. Needle depression converts the tension pneumothorax into an open pneumothorax; needle decompression is a temporizing measure and should be followed promptly with tube thoracostomy. If the patient’s hemodynamics fail to improve following decompression, consider other causes of hypoperfusion, including pericardial tamponade.
FIGURE 261-3.
Decompression of a tension pneumothorax with a catheter-over-the-needle. A. The catheter-over-the-needle is inserted through the second intercostal space and into the pleural cavity. B. The catheter is advanced while the needle is removed. [Reproduced with permission from Reichman EF (ed): Emergency Medicine Procedures, 2nd ed. © 2013. McGraw-Hill Education, New York, NY. Fig 38-2A&B.]
Common causes of massive hemothorax include injury to the lung parenchyma, intercostal arteries, or internal mammary arteries. Each hemithorax can hold 40% of a patient’s circulating blood volume. A massive hemothorax is defined in the adult as at least 1500 mL. Massive hemothorax is life threatening by three mechanisms. First, acute hypovolemia does not allow for sufficient preload to sustain left ventricular function and adequate cardiac output. Second, the collapsed lung results in hypoxia by creating alveolar hypoventilation, ventilation–perfusion mismatch, and anatomic shunting. Third, the hydrostatic pressure of the hemothorax compresses the vena cava and the pulmonary parenchyma, further impairing preload and raising pulmonary vascular resistance, respectively. Although the clinical signs and symptoms of hemothorax in the chest trauma patient can vary, suggestive findings include decreased or absent breath sounds and no chest movement with respiratory effort.
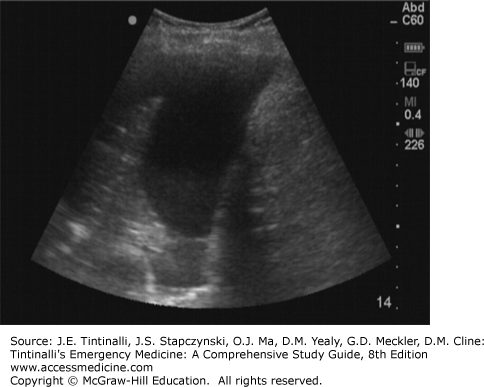
The diagnosis is made by plain chest radiography when complete opacification of a hemithorax is observed. As an alternative to chest radiography in the unsTable patient, point-of-care US may reveal a layer of fluid between the chest wall and lung (Figure 261-4). Lung collapse, due to intubation of the contralateral mainstem bronchus, can mimic the appearance of hemothorax; always verify proper endotracheal tube placement. Treatment is tube thoracostomy and replacement of blood products as clinically indicated.
Open pneumothorax is a communication between the pleural space and surrounding atmospheric pressure. This is sometimes referred to as a “sucking chest wound,” but also may be due to small rents in the parietal pleura or small air passages without an obvious penetrating injury. Respiratory distress is due to lung collapse and subsequent inability to ventilate the affected lung. Air entry and breath sounds are often diminished on the affected side, and chest wall motion can be impaired. The initial therapeutic maneuver to treat a sucking chest wound is to cover the wound with a three-sided dressing such that air can exit but not enter the chest. Avoid complete occlusion, as this may convert the injury into a tension pneumothorax. Do not insert a chest tube through the trauma wound, as it is likely to follow the missile or knife tract into the lung or diaphragm.
Systemic air embolism is an acute complication of severe chest trauma and presents with disastrous circulatory and cerebral complications. Patients with penetrating chest wounds who require positive-pressure ventilation are at risk for developing air embolus. High ventilatory pressures, especially >50 cm H2O, may force air from an injured bronchus into an adjacent injured vessel. Air embolus may lead to severe dysrhythmias or CNS deficits. Patients presenting with hemoptysis in the setting of penetrating chest trauma are at particular risk for this serious complication.
If systemic air embolism is suspected or diagnosed, place the patient in a flat supine position with 100% oxygen applied, which may decrease air bubble size by displacing nitrogen and promoting resorption of the embolus. There is no evidence to support the theoretical benefit of the Trendelenburg (head down) position in arterial air embolism. Hyperbaric oxygen therapy, if available, helps to decrease size and increase resorption of air bubbles. Airway management of patients at risk for systemic air embolism should include maneuvers that can selectively ventilate each lung. In unilateral lung injury, isolating and ventilating the uninjured lung can, in theory, be used to prevent systemic air embolism. In the event of circulatory collapse, treatment begins with cardiopulmonary resuscitation protocols and an immediate thoracotomy to clamp the injured area of lung. This is followed by air aspiration from the heart and ascending aorta.17,18 Open cardiac massage with clamping of the ascending aorta may help push air through the coronary arteries. Initiate cardiopulmonary bypass promptly, if available.
TUBE THORACOSTOMY
Evacuate large hemothoraces or hemopneumothoraces as rapidly as possible to decrease their negative effects on ventilation and perfusion. Double-check clinical and radiographic findings to make sure you are placing the chest tube on the proper side of the patient. Historically, a 24F or 28F (8.0 or 9.3 mm) chest tube was recommended for a simple pneumothorax and a >32F (>10.7 mm) chest tube for a suspected hemothorax. One study suggests there are no differences in efficacy of drainage, complication rate, pain, or need for additional procedures when using small or large chest tubes.19
Perform tube thoracostomy for treatment of traumatic pneumothorax or hemothorax in the anterior axillary line at the level of the nipple in men or inframammary crease in women (corresponding to the fifth intercostal space) just behind the lateral edge of the pectoralis major.
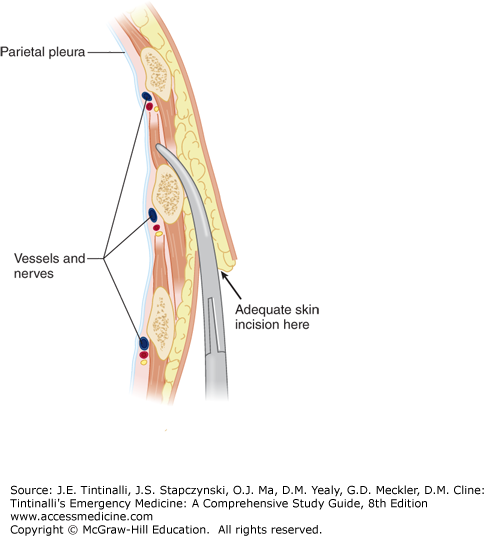
Make an oblique skin incision at least 1 to 2 cm below the interspace through which the tube will be placed. Insert a large clamp through the skin incision and into the muscles in the next higher intercostal space, just above the rib, thus avoiding the neurovascular bundle (Figure 261-5). The resulting oblique tunnel through the subcutaneous tissue and intercostal muscles usually closes promptly after the chest tube is removed, thereby reducing the chances of recurrent pneumothorax.
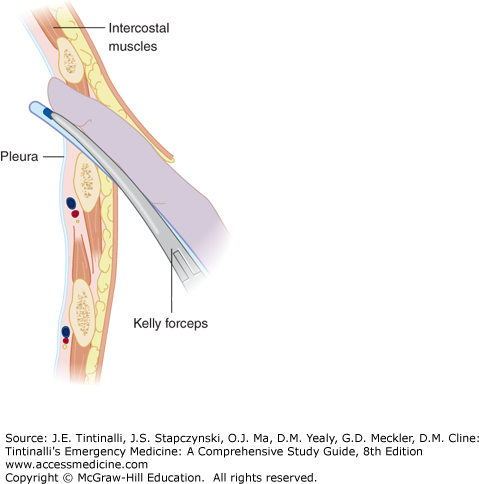
Once the clamp is pushed through the internal intercostal fascia, open it to enlarge the hole to at least 2.0 cm. Insert a finger along the top of the clamp through the hole to verify the position within the thorax and to verify that the lung is not adhering to the chest wall (Figure 261-6).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree