First author
Year
Incidence of VAP in ARDS patients
Incidence of VAP in no ARDS patients
Diagnostic methods
Journal
Markowicz
2000
49 out of 134 (36%)
173 out of 744 (23%)
PSB, BAL, or mini-BAL
Am J Respir Crit Care Med Vol 161. pp. 1942–1948, 2000
Meduri
1998
40 out of 94 (43%)
NA
Bilateral BAL
Am J Respir Crit Care Med Vol 158. pp. 870–875, 1998
Chastre
1998
31 out of 56 (55%)
53 out of 187 (28%)
PSB and BAL (before any change in antibiotic treatment)
Am J Respir Crit Care Med Vol 157. pp. 1165–1172, 1998
Delclaux
1997
24 out of 30 (60%)
NA
Mini-BAL
Am J Respir Crit Care Med Vol. 156. pp. 1092–1098, 1997
Sutherland
1995
16 out of 105 (15%)
NA
PSB, BAL
20.3 Morbidity and Mortality
ARDS is one of the most severe pulmonary diseases, leading to significant patient morbidity and healthcare burden [7]. Thus, it is expected that any iatrogenic complication that ensues during the course of ARDS may delay weaning from the MV, prolong the intensive care unit (ICU) stay, require additional treatments, and exponentially increase costs. In a recent international, multicenter, prospective cohort study of 2377 patients with ARDS, the median length of MV was 8 days (interquartile range (IQR), 4–16). Early studies have consistently reported a significant increase in duration of MV, when VAP occurred in ARDS patients. Chastre et al. found that in ARDS patients who developed VAP, the length of MV increased from 17 ± 19 to 34 ± 32 days, whereas Markowicz et al. found that in ARDS patients, the first episode of VAP did not occur until a mean length of MV of 11.7 ± 11.9 days. Interestingly, the mean duration of MV, for ARDS patients who did not develop VAP, was highly similar: 11.3 ± 9.1 days. Conversely, when they did develop VAP, the length of stay increased up to 33 ± 21 days.
It is fascinating that in early studies [6, 8], investigators failed to demonstrate any impact of VAP on mortality. Considering the clinical severity of ARDS, it is somewhat difficult to justify the lack of effect on mortality, when VAP complicates the course of the disease. Yet, several factors may explain these counterintuitive findings. First, in ARDS patients, the occurrence of VAP can be biased by the presence of competing events, specifically death or ICU discharge, which preclude VAP occurrence or dramatically changing its risk [9]. For instance, a highly severe ARDS patient, at high risk of VAP, could decease during the time on MV, but before the onset of VAP. Unfortunately, traditional methods of time-to-event analysis considered patients who died while on MV as non-informative censoring, resulting in biased estimates. Yet, in more recent analyses [10–12], patients have been considered under risk either up to the occurrence of VAP or until one of the other competing events occurred or until follow-up was completed. Thus, latest studies [10], in patients without ARDS, reported an attributable mortality of 10%. Also, it seems that surgical patients and with midrange severity of illness present the highest associated risk of mortality. To the best of our knowledge, no studies have been specifically assessed the attributable mortality of VAP in ARDS patients. Nevertheless, considering the severity of this condition, it is rationale to assume that VAP might unhinge the delicate clinical balance of these patients, and lead to worse outcome.
20.4 Pathogenesis
ARDS patients on MV often become colonized by exogenous pathogens acquired from healthcare personnel or, more commonly, by endogenous pathogens that colonize the gastrointestinal tract, oropharynx, tracheal tube, and proximal trachea. Several defense mechanisms prevent overwhelming colonization, i.e., cough, mucus clearance, and cellular and humoral immune responses. Yet, in ARDS patients, these defenses are impaired, because of the severe lung injury, comorbidities, and tracheal intubation. In the next paragraphs, several risk factors for the development of VAP will be described, with a specific focus on the specific risks and conditions that may occur in ARDS patients.
20.4.1 The Endotracheal Tube and Pulmonary Aspiration
The primary mechanism in the pathogenesis of VAP is through pulmonary aspiration of colonized oropharyngeal secretions across the high-volume low-pressure (HVLP) endotracheal tube (ETT) cuff. The HVLP cuff was designed in the late 1970s to closely monitor the pressure exerted against the trachea [13]. Yet, when HVLP cuffs are inflated within the trachea, folds are formed, because the cuff outer diameter is larger than the tracheal internal diameter [14]. In vitro studies have demonstrated that cuff outer diameter, length, and positive end-expiratory pressure (PEEP) are the main determinants of cuff sealing efficacy. Indeed, the larger the cuff the more folds form on its surface, promoting leakage across the folds (Fig. 20.1).
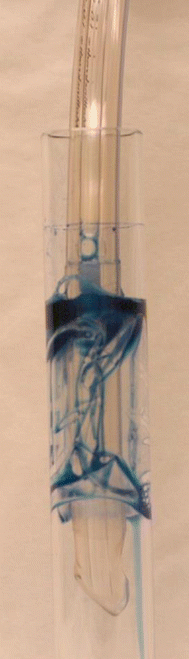

Fig. 20.1
High-volume low-pressure cuff inflated within an artificial translucent tracheal model. The tracheal model internal diameter is 20 mm, whereas the cuff outer diameter is 28 mm. Upon inflation, folds are formed on the cuff surface. Methylene blue is poured above the cuff to highlight leakage of fluid across the channels formed by the folds
Pathogens may also grow on the internal surface of the ETT, forming a complex structure called biofilm [15]. ETT biofilm is composed by sessile bacteria within a self-produced exopolysaccharide matrix and respiratory secretions [16–18]. It is still not fully elucidated the role of ETT biofilm in the pathogenesis of VAP. Shah and collaborators [19] demonstrated a close association between intraluminal narrowing of the ETT by biofilm/secretions and length of stay on MV. Given that ARDS patients present longer periods of MV, it is reasonable to assume that a large amount of biofilm builds up and constitutes a persistent source of infection for recurrent episodes of VAP.
20.4.2 Oropharyngeal Colonization
Overall, in critically ill patients on MV, the oral flora shifts early to a predominance of aerobic Gram-negative pathogens [20], Pseudomonas aeruginosa, and methicillin-resistant Staphylococcus aureus (MRSA). Comorbidities and inherent patient’s characteristics such as alcohol abuse [21–23], diabetes [24, 25], and chronic obstructive pulmonary disease [26] increase risks of colonization by Gram-negative pathogens. ARDS patients present additional risk factors, because they require prolonged periods of MV and large amounts of antibiotics. The ETT is a foreign body, and after several days within the mouth, the salivary flow [27] and oral pH progressively decrease. This leads to an abnormal adherence of pathogens to the buccal epithelial cells [28]. These risks are amplified in patients with poor oral health upon intubation [29–31]. As mentioned above, oropharyngeal pathogens are ultimately aspirated into the airways and may ultimately cause VAP [32].
20.4.2.1 Gastrointestinal Tract
In the 1990s, several pivotal studies demonstrated that the stomach of ICU patients is colonized by pathogens, due to alkalinization of gastric contents by enteral nutrition and drugs for stress ulcer prophylaxis [33]. Commonly, ARDS patients are on enteral feeding and receive these prophylactic drugs that alter the gastric pH. Gastric pathogens may translocate into the oropharynx, due to the gastroesophageal reflux. In particular, prone position could increase risks for oropharyngeal colonization by gastric pathogens. To date, no studies have assessed gastroesophageal reflux in the prone position, but considering that in previous studies [34, 35], vomiting was a frequent complication, gastroesophageal reflux may be common in such position, particularly during enteral feeding.
20.4.3 Other Risk Factors
In ARDS patients, several other risk factors may play a role in the pathogenesis of VAP. ARDS patients require a high level of care throughout the day; as a result, the risks for cross transmission of pathogens from other patients are exponentially increased. This may be relevant in ICUs where patient-to-nurse ratios are higher than 1, where healthcare personnel are not adequately trained on infection control and preventive strategies, and where strict sterilization protocols and handwashing with alcohol-based solutions are not efficiently implemented.
Mucociliary clearance is one of the most important innate airway defense mechanisms to clear pathogens. In young, healthy nonsmokers, the mucociliary velocity ranges between 10 and 15 mm/min. Studies on intubated critically ill patients have confirmed a tenfold decrease in mucociliary clearance rate [36] and higher risks of VAP in patients with the slowest rates. In ARDS patients, several factors may affect mucus clearance rate. First, endotracheal intubation and the inflation of the cuff have shown a drastic decrease in mucus clearance rate within hours [37]. Second, ARDS patients often require high concentration of oxygen, which is known to reduce mucus clearance rate in a dose-dependent fashion [38]. Finally, these patients often present markedly increased respiratory drive that impairs efficiency of commonly used humidifiers. Of note, even brief period of suboptimal humidification may depress cilia function [39].
Finally, investigators have found a temporary immunoparalysis early in the course of the critical illness and admission to the ICU [40], which is the results of various and heterogeneous inciting events like ARDS [41], severe trauma [42], and sepsis [43]. The immunoparalysis is aimed at counterbalancing the exaggerated inflammatory response with an anti-inflammatory milieu. Yet, during this period of immunodepression, the organism is unable to adequately respond to a second hit, increasing potential risks of acquiring iatrogenic infections, such as VAP.
20.4.4 Etiologic Agents
ARDS patients are at higher risk for developing VAP caused by S. aureus; non-glucose-fermenting Gram-negative bacilli, i.e., P. aeruginosa, Acinetobacter baumannii, and Enterobacteriaceae. Of note, in ARDS patients, antibiotics are profusely administered very early. As a result, antimicrobial causes a selective pressure over more susceptible bacteria, allowing potentially resistant bacteria to survive. In particular, Chastre et al. [6] compared VAP in patients with and without ARDS and found that methicillin-resistant S. aureus was isolated in 44% and 17% of the patients with or without ARDS, whereas Markowicz et al. [8] found that non-fermenting Gram-negative bacilli were more frequent in ARDS patients (47% vs. 33% in non-ARDS patients). Also, methicillin-susceptible S. aureus was less common in ARDS patients (3% vs. 15% in non-ARDS patients). In ARDS patients, VAP is often caused by multiple pathogens [44, 45]. Combes and colleagues [46] studied 124 ICU patients, of whom 65 (52%) had monomicrobial VAP and 59 (48%) had polymicrobial VAP. In most patients (34%), two different bacteria were isolated; however, up to four different bacteria coexisted in seven patients (6%). Interestingly, no differences were detected in mortality rates at 30 days between patients with polymicrobial or monomicrobial infection. Also, multiple episodes of VAP may occur in approximately 50% of the patients, which may be related to the prolonged stay in the ICU or substantial presence of ETT biofilm.
20.5 Prevention
VAP is an iatrogenic infection that, in ARDS patients, impairs morbidity and constitutes an important burden for the healthcare system. Therefore, preventive strategies should be applied to avoid the disease. To date, several preventive measures are available for all mechanically ventilated patients and are often grouped as a bundle (Table 20.2). Nevertheless, in the next paragraphs, the most important strategies aimed at preventing VAP in ARDS patients will be highlighted.
Table 20.2
Preventive bundle
Implementation of educational programs for caregivers and frequent performance feedbacks and compliance assessment Strict alcohol-based hand hygiene |
Daily sedation vacation and implementation of weaning protocols |
No ventilatory circuit tube changes unless the circuit is soiled or damaged |
Use of tracheal tube with cuff made of novel materials |
Use of silver-coated tracheal tube |
Application of low-level PEEP during tracheal intubation |
Aspiration of subglottic secretions |
Internal cuff pressure maintained within the recommended range and carefully controlled during transport of patients outside ICU |
Oral care with chlorhexidine |
Avoid stress ulcer prophylaxis in very low-risk patients for gastrointestinal bleed, and consider the use of sucralfate when indicated |
Semirecumbent patient positioning |
SDD for patients requiring >48 h of mechanical ventilation |
20.5.1 Patient Transport and Sedation
ARDS patients often require intrahospital transport out of the ICU for surgical procedures, imaging, and invasive interventions. However, transport out of the ICU has been associated with potential complications, primarily VAP [47]. Kollef et al. [48] studied 273 patients, who required at least one transport outside the ICU vs. 248 who did not. Of note, 66 (24.2%) of the transported patients developed VAP, compared with 11 (4.4%) of the patients who were not trasported outside (relative risk = 5.5; 95% confidence interval [CI] = 2.9–10.1; p < 0.001). Importantly, prior to the transport, clinicians and nursing staff should carefully check the internal pressure of the ETT cuff, particularly when the patient is expected to be maintained supine during diagnostic or therapeutic procedures; ventilator circuits should be carefully manipulated in order to prevent aspiration of colonized fluids from within the circuit. Also, sedation should be increased only if necessary to improve patient safety or for a specific test that requires immobility. Yet, sedation should be rapidly interrupted or lightened as soon as possible. Along the same line, even in patients with ARDS, daily interruption or lightening of sedation [49–51] and early mobilization [52] should be attempted to avoid severe impairment of respiratory defenses and prolonged intubation.
20.5.2 Extracorporeal Membrane Oxygenation and Tracheal Extubation
Tracheal intubation is the main risk factor for VAP and therefore should be avoided whenever possible or used for the shortest time possible. Very recently, extracorporeal membrane oxygenation (ECMO) has been used in awake ARDS patients who were extubated and allowed to breathe spontaneously [53]. In an interesting preliminary study, Hoeper MM et al. [54] studied six ARDS immunocompromised patients with only lung failure, who underwent an “awake ECMO” strategy, through extubation shortly after initiation of ECMO. Overall, 66% of the patients survived. Only one patient developed VAP, but after being re-intubated for complications. These fascinating results call for future larger studies to avoid tracheal intubation, which is the main culprit of the disease.
Novel Endotracheal Tubes
In the latest years, several improvements in the design of ETT have been achieved, specifically to address HVLP cuff limitations. We recently demonstrated [14] that the main determinants in the design of ETT cuff for sealing effectiveness are the cuff material, outer diameter, length, and the internal cuff pressure. In particular, narrower cuffs form less folds, upon inflation within the trachea. Cuffs made of new materials such as polyurethane [55] have been developed and tested in laboratory and clinical trials. A few studies in medical ICU patients [56, 57] or cardiac surgical patients [58, 59] have shown that polyurethane cuffs reduce risks of developing VAP. Yet, in a recent multicenter study [60], cuffs composed of cylindrical polyvinyl chloride, cylindrical polyurethane, conical polyvinyl chloride, or conical polyurethane were compared for the prevention of tracheal colonization, and no differences were found. Importantly, the majority of aforementioned studies used novel ETTs in all patients, irrespective of their inherent risks of developing VAP. Conversely, we think that their use should only be limited to the patients at very high risk. In particular, to date, no studies have tested novel ETTs in ARDS patients, who present the highest risk factors for VAP.
In ARDS patients who are often ventilated with high level of positive end-expiratory pressure (PEEP), it could be assumed that aspiration of oropharyngeal secretions across the cuff is largely counterbalanced by PEEP. Lucangelo et al. [61] investigated the effects of 5–8 cmH2O of PEEP in normoxemic ventilated patients. They showed a significant reduction in the rate of VAP (PEEP group 9.4%, control patients 25.4%, relative risk, 0.37; 95% CI = 0.15–0.84; p = 0.017). Thus, in the absence of major contraindication, a low level of PEEP should always be maintained in ARDS patients, even during the weaning period to avoid pulmonary aspiration. It is also very important to maintain the internal ETT cuff pressure between 25 and 30 cmH2O to prevent aspiration of contaminated secretions into the lower airways or tracheal injury. Ideally, in ARDS patients at high risk of VAP, it would be advisable to use continuous control of internal cuff pressure, as demonstrated in previous studies [62–64], to reduce risks of unforeseen cuff deflation and pulmonary aspiration.
Some ETTs allow aspiration of colonized subglottic secretions above the cuff and potentially prevent macro-leakage into the trachea. A recent meta-analysis [65], pooling data from 17 randomized clinical trials of a total of 3369 patients, has shown that subglottic secretion drainage reduced the overall risk ratio (RR) for VAP by half (risk ratio, 0.58; 95% CI, 0.51–0.67; I2 = 0%). Most of the studies were conducted in post-cardiac surgery patients; thus, although this strategy seems highly effective, further evidence on ARDS patients is needed before wider use.
Finally, the use of ETT coated with antimicrobial agents such as silver [66] could be another promising strategy to prevent biofilm formation and repetitive episodes of VAP. Unfortunately, to date, only one large randomized clinical trial, the North American Silver-Coated Endotracheal Tube (NASCENT) randomized trial [67], evaluated benefits of a silver-coated vs. a conventional tube. They included patients with lower degrees of severity, and they found a lower incidence of microbiologically confirmed VAP (37/766 (4.8%) vs. 56/743 (7.5%); p = 0.03), for a relative risk reduction of 35.9%. Thus, the benefits of coated ETTs in ARDS patients at high risk of VAP should also be investigated in future studies.
Body Position
Nowadays, most of the ICU patients are kept in the semirecumbent position, because intubated patients are at higher risk for gastropulmonary aspiration when placed in the supine position (0 degree), as compared with a semirecumbent position (45 degrees) [33, 68, 69]. One randomized trial [70] demonstrated a reduction in the incidence of VAP in patients positioned in the semirecumbent position, compared with patients completely supine. Interestingly, as clearly demonstrated by Guerin et al. [71] prone position is highly beneficial in ARDS patients. Theoretically, the prone position could also have VAP preventive effects, because patients, as soon as they are placed to the prone position, clear from the ETT and the mouth all secretions that are retained. A study by Ayzac [72] and collaborators retrospectively evaluated patients included in their early prone-supine study the impact on VAP. Unfortunately, they found that the cumulative probability of VAP was higher in the prone than in the supine position group (46.5% at 90 days in the prone position group and 33.5% in the supine position group (p = 0.11)).
20.6 Diagnosis
In mechanically ventilated patients, VAP is suspected when a new radiographic infiltrate develops with several unspecific clinical signs [73, 74] of pulmonary infection, such as fever or hypothermia, purulent secretions, and leukocytosis or leukopenia. Unfortunately, in ARDS patients, chest radiographs are difficult to interpret (Fig. 20.2), because bilateral infiltrates are already present, prior to the development of VAP. Also, it is difficult to differentiate among cardiogenic and non-cardiogenic pulmonary edema, pulmonary contusion, atelectasis, and pneumonia. A few studies appraised VAP diagnostic accuracy of portable chest radiographs in the ICU [75–79]. Wunderink et al. [78] demonstrated that in deceased patients with autopsy-proven VAP, no single radiographic sign had a diagnostic accuracy greater than 68%. In ARDS patients, not even the presence of air bronchograms or alveolar opacities improved VAP diagnostic accuracy.
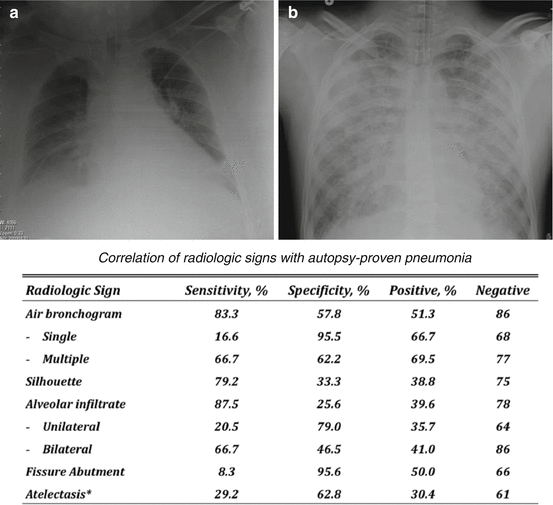

Fig. 20.2
The challenges in the diagnosis of ventilator-associated pneumonia through analyses of portable chest radiographs. A: patient who developed clinical signs of ventilator-associated pneumonia after 3 days of mechanical ventilation, and, at the chest radiograph, a new progressive pulmonary infiltrate was evident at the lower right lobe. B: patient with acute respiratory distress syndrome, who developed clinical signs of ventilator-associated pneumonia after 7 days of mechanical ventilation. Of note, due to the bilateral infiltrates, it is challenging to identify any new pulmonary infiltrate. At the bottom of the figure is reported specificity and sensitivity of radiologic signs corroborated by gross findings upon autopsy (From Wunderink et al. [78])
The Clinical Pulmonary Infection Score (CPIS) was developed to improve diagnostic accuracy [80]. CPIS comprises clinical variables, such as temperature, blood leukocyte count, volume and purulence of tracheal secretions, oxygenation, pulmonary radiographic findings, and semiquantitative culture of tracheal aspirate. In pivotal studies [80, 81], a CPIS value ≥ 6 was an accurate threshold to identify patients with VAP. Yet, in ARDS patients, the clinical value of CPIS remains to be validated.
In the latest guidelines for the management of patients with VAP [82], it is strongly recommended to obtain cultures of respiratory secretions from all patients with clinical suspicion of VAP. Nevertheless, considering that available evidence [83–87] demonstrated that invasive quantitative sampling did not impact any clinical outcome, including mean duration of MV, ICU length of stay, or mortality [88], the specimens can be obtained non-invasively, and cultures can be performed semiquantitatively. Many sampling procedures of respiratory secretions, such as endotracheal aspirates, BAL, and protected specimen brush (PSB), are available. Previous studies in patients with ARDS have used BAL, mini-BAL, and PSB to diagnose VAP. In addition, there are several microbiological techniques including Gram staining and intracellular organism count from specimens obtained via BAL. Each diagnostic technique has advantages, as well as limitations and provides different diagnostic specificity/sensitivity. Overall, qualitative cultures of endotracheal aspirates have, potentially, a high percentage of false-positive results, due to frequent bacterial colonization of the proximal airways. Conversely, quantitative cultures of distal samples have, theoretically, an improved specificity but worse sensitivity.
In an important study by Meduri and collaborators [89], the diagnostic accuracy of bilateral BAL sampling was assessed in ARDS patients with clinical suspicion of VAP. Among 55 bronchoscopies that yielded positive culture results, 33 (60%) had significant growth in only one lung. Bilateral growth in BAL samples was more likely to be polymicrobial, and with a bacterial growth >105 cfu/ml. The authors also corroborated changes in BAL accuracy, when antibiotics were administered before BAL sampling.
In conclusion, in ARDS patients with clinical suspicion of VAP, sampling of the lower respiratory tract is advisable to accurately diagnose VAP and appropriately use the most effective antibiotics, after microbiology results become available. Microbiological diagnosis of VAP is pivotal not only for determining whether a patient has VAP, but also for narrowing or discontinuing antimicrobial therapy as soon as possible. Considering that VAP in ARDS patients is multifocal, predominantly in lower lobes [90] and it is bilateral in 40% of the cases, non-bronchoscopic techniques are sufficiently reliable to obtain respiratory secretions. This also in light of the potential complications that may occur in ARDS patients during bronchoscopy.
20.7 Treatment
A major problem in the management of patients with suspicion of VAP is when to initiate antibiotics and the prospective susceptibility of the causative pathogens. The indiscriminate and empiric administration of antibiotics, following clinical suspicion, contributes to the emergence of multidrug-resistant (MDR) pathogens and exposes the patient to antibiotic-related adverse effects and higher costs [91]. On the other hand, it is mandatory to initiate prompt treatment of VAP to improve survival [92–95]. However, the choice of the initial antibiotic treatment is challenging, particularly in ARDS patients undergoing long-term antibiotic treatment before VAP development [96]. Also, it is important to consider patient’s inherent risk factors for MDR pathogens (Table 20.3) [82]. Finally, physicians should consider resistance patterns, which vary extremely among countries, regions, hospitals, and ICUs. Rello et al. [97] analyzed variations in VAP etiology among three Spanish ICUs and compared them with data collected in Paris. The authors concluded that VAP pathogens largely changed among the four clinical centers, with marked differences among the microorganisms isolated from the Spanish and French centers. Therefore, clinicians must be aware of the most common microorganisms in their own unit and antimicrobial susceptibility to avoid the administration of inadequate empiric antimicrobial therapy.
Table 20.3
Risk factors for ventilator-associated pneumonia caused by multi-drug resistant pathogens |
Prior intravenous antibiotic use within 90 days |
Septic shock upon diagnosis of VAP |
ARDS preceding VAP |
Five or more days of hospitalization prior to the occurrence of VAP |
Acute renal replacement therapy prior to VAP onset |
Risk factors for methicillin-resistant Staphylococcus aureus |
Prior intravenous antibiotic use within 90 days
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|


