PULMONARY EMERGENCIES
KYLE NELSON, MD, MPH, MARK I. NEUMAN, MD, MPH, AND JOSHUA NAGLER, MD, MHPEd
GOALS OF EMERGENCY THERAPY
Pulmonary emergencies are common in children, accounting for approximately 10% of pediatric emergency department (ED) visits and 20% of hospitalizations. Significant respiratory distress, particularly impending respiratory failure, must be promptly recognized and effectively managed. General approaches to improve oxygenation and ventilation are combined with therapy tailored to the underlying condition. The differential diagnosis varies by age, with many conditions unique to specific pediatric age groups. National guidelines are published for evaluation and management of asthma, bronchiolitis, and pneumonia, which have informed local clinical practice guidelines and development of measurable outcomes reflecting quality of care.
KEY POINTS
 An age-appropriate differential diagnosis must be considered when managing respiratory distress.
An age-appropriate differential diagnosis must be considered when managing respiratory distress.
 Normal vital sign ranges vary according to the age of the child.
Normal vital sign ranges vary according to the age of the child.
 Children with severe respiratory distress may rapidly decompensate and progress to respiratory failure.
Children with severe respiratory distress may rapidly decompensate and progress to respiratory failure.
RELATED CHAPTERS
Signs and Symptoms
Clinical Pathways
• Pneumonia, Community-Acquired: Chapter 90
Medical, Surgical, and Trauma Emergencies
• Allergic Emergencies: Chapter 93
• Thoracic Trauma: Chapter 123
ACUTE RESPIRATORY FAILURE
CLINICAL PEARLS AND PITFALLS
• Impending respiratory failure must be promptly recognized and managed. Effective early intervention can limit progression, morbidity, and mortality.
• A systematic approach to prioritizing assessment and support of airway, breathing, and circulation should be employed.
• Emergency management of acute respiratory failure often involves both diagnostic testing and lifesaving therapeutic maneuvers.
• After stabilization, attention must be given to treating the underlying condition.
Current Evidence
While many cases of respiratory distress are benign and self-limited, requiring minimal or no intervention, pulmonary diseases contribute to significant morbidity and mortality in pediatrics, including 3% to 5% of deaths; some of these deaths may be preventable. Importantly, respiratory failure often precedes cardiopulmonary arrest in children; unlike adults for whom primary cardiac disease is often responsible. Therefore, careful assessment of cardiopulmonary status and anticipation of and preparation for deterioration are important aspects of care. Prompt recognition and treatment of impending respiratory failure can be lifesaving and may reduce morbidity and mortality.
By definition, there are two components to respiratory failure—inability of the respiratory system to (1) provide sufficient oxygen for metabolic needs (hypoxic respiratory failure) and (2) excrete the carbon dioxide (CO2) produced by the body (hypercapnic or ventilatory respiratory failure). Both are often present simultaneously, but to varying degrees.
Causes of acute respiratory failure can also be categorized with consideration of location in the respiratory system (Table 107.1). In addition to primary pulmonary disease, many disorders outside the respiratory tract can lead to respiratory failure.
Primary pulmonary conditions must be considered. Parenchymal lung disease can lead to acute respiratory failure, particularly in younger children, and those with underlying cardiopulmonary disease (e.g., bronchopulmonary dysplasia [BPD] or congenital heart disease). In such cases, the additional respiratory embarrassment from acute pulmonary infection can induce respiratory failure.
Any condition that causes further narrowing or collapse of the intrinsically small pediatric airway can have a profound effect on air flow. Edema from infectious, allergic, or caustic etiologies; foreign material in the airway; or obstruction by enlarged or compressing anatomic structures can restrict airflow. These may occur in isolation or in combination.
Asthma is the most common etiology for lower airway disease, but infections such as bronchiolitis or viral pneumonia are also common. Foreign-body aspiration can present acutely with airway obstruction, or may be a delayed diagnosis following the development of secondary postobstructive atelectasis or pneumonia.
TABLE 107.1
CAUSES OF ACUTE RESPIRATORY FAILURE IN CHILDREN
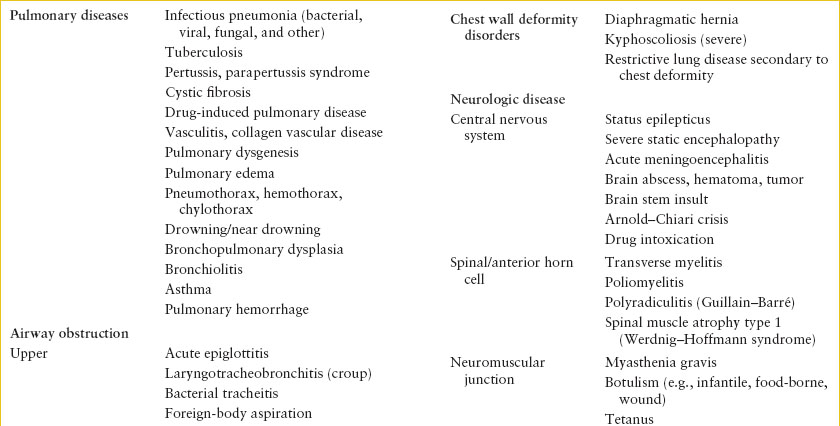

Chest wall deformities and mechanical impairments prevent full expansion of the chest, leading to decreased vital capacity, decreased minute ventilation, and resultant hypercapnia. Inefficient respiratory efforts can cause subsequent hypoxia. Oftentimes, these patients maintain near normal respiratory function until additional physiologic compromise occurs, often from illness as minor as an upper respiratory infection.
Disruption of nonpulmonary respiratory physiology often results from either reversible or irreversible causes of central nervous system (CNS) disease. CNS disease may result in depressed respiratory drive, or inability to maintain protective airway reflexes. Alternatively, neurologic disease may directly affect the peripheral nerves or muscles, leading to either airway obstruction or inadequate excursion of the chest wall and diaphragm. The result is inadequate gas exchange and ventilation–perfusion (V/Q) mismatch.
Finally, numerous other nonpulmonary diseases may precipitate respiratory failure. Though with varied underlying pathophysiology, the diseases listed in Table 107.1 may alter the balance of O2 consumption and CO2 production such that gas exchange cannot be maintained by the respiratory system, leading to secondary respiratory failure.
Goals of Treatment
The goals of management of acute respiratory failure are correction of hypoxia and sufficient support of ventilation. Immediate efforts should be directed toward both lifesaving maneuvers and appropriate diagnostic testing, as establishing a diagnosis will inform disease-specific management.
Clinical Considerations
Clinical Recognition
Acute respiratory failure represents the severe end of the spectrum of respiratory disease. Though the onset can be hyperacute (e.g., complete airway obstruction from foreign-body aspiration or traumatic injury to phrenic nerve with complete loss of respiratory effort), respiratory failure more commonly results from a progression of respiratory illness and distress. The differential diagnosis is broad, though the underlying causes vary by age. While congenital anomalies are likely to present in the first several months of life, some may present in older infants and toddlers. Some progressive neurologic conditions may present in older children. It is important to appreciate that normal ranges of respiratory rate differ by age (Table 107.2). Some cases may involve patients without concerning medical history that have a severe acute condition such as upper airway obstruction from aspirated foreign body or swelling due to infection. Some cases may involve progressive deterioration of a chronic condition such as cystic fibrosis (CF). Details of management differ according to acute diagnosis and pathophysiology of underlying condition.
Patients at risk for acute respiratory failure must be quickly identified and managed to prevent deterioration. In general, patients presenting with significant respiratory distress (i.e., grunting, gasping, and severely increased work of breathing) are at risk for respiratory failure. Additionally, neonates, patients with cardiac disease, and those with worrisome trajectories and/or tiring despite therapy are considered at risk of respiratory failure. Other concerning clinical findings, blood gas abnormalities, and pulmonary function abnormalities commonly present in the setting of respiratory failure are listed in Table 107.3. Appreciation of complicating underlying conditions and the current clinical status, including response to chronic therapy and details of prior exacerbations will help to assess those at risk of deteriorating respiratory failure and inform treatment decisions.
TABLE 107.2
RESTING RESPIRATORY RATE BY AGE

TABLE 107.3
DIAGNOSIS OF ACUTE RESPIRATORY FAILURE FROM PULMONARY CAUSES IN CHILDREN

Triage
Children with signs of impending or acute respiratory failure should be rapidly identified based on appearance or vital signs and immediately evaluated with attention to necessary lifesaving maneuvers. Supplemental oxygen and support of ventilation should be provided emergently as indicated, while additional efforts to determine underlying etiology are being addressed.
Initial Assessment/H&P
Diagnosis of acute respiratory failure is commonly made clinically, though laboratory or pulmonary function testing can be supportive. Initial assessment involves prompt appraisal of the child’s appearance, level of alertness, airway patency, breathing effort, and circulation. Resuscitative efforts may be necessary to clear or support an obstructed airway, provide oxygen, and support effective ventilation. Initial history should be brief, focused, and succinct. One approach to consider is the “AMPLE” history, which involves queries into allergies, medications, pertinent medical history, last meal, and events involved in present illness including treatments already administered.
Patients with acute respiratory failure should be continually assessed using cardiopulmonary monitoring of heart rate, cardiac rhythm, respiratory rate, pulse oximetry, and blood pressure. Noninvasive monitoring of end-tidal carbon dioxide (ETCO2) (i.e., capnography) is also an important adjunct, providing information about ventilatory status, including adequacy of assisted ventilation if performed.
Management
Management of acute respiratory failure is critical care in the ED. It involves performing necessary therapeutic interventions to assist oxygenation and ventilation along with close monitoring for further deterioration and consideration of appropriate diagnostic testing (Table 107.4).
Supplemental oxygen should be provided during initial assessment and any resuscitative efforts. This is most appropriately accomplished using a nonrebreather mask for spontaneously breathing patients. The goal oxygen saturation percentage may vary according to underlying and suspected acute conditions, but >90% is an appropriate initial goal for most patients. While some patients with cardiac disease may not tolerate high amounts of supplemental oxygen depending on their physiology, in general, immediate lifesaving efforts should include provision of supplemental oxygen while further details of the condition are sought and risks of overoxygenating are considered.
TABLE 107.4
MANAGEMENT OF ACUTE RESPIRATORY FAILURE
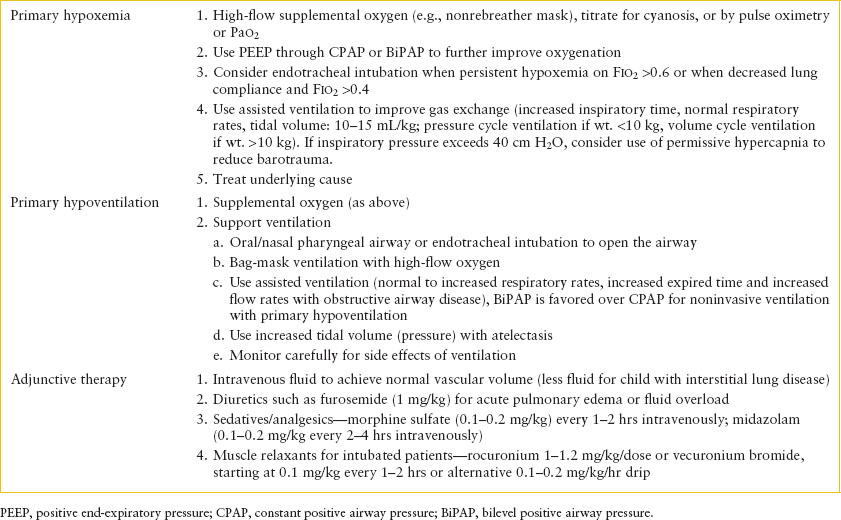
Clinicians should be adept at assessing airway patency and performing emergency maneuvers to optimize oxygen delivery and assisted ventilation. Use of a flow-inflating resuscitation bag (aka “anesthesia bag”) can deliver 100% oxygen and continuous positive airway pressure (CPAP). Positive-pressure breaths utilizing a self-inflating or flow-inflating bag will further increase oxygen delivery. Importantly, CPAP cannot be delivered though a self-inflating (Ambu) bag. Endotracheal intubation provides the most effective means of increasing Pao2 and is required for patients with persistent hypoxemia despite other interventions including noninvasive ventilation support such as CPAP or BiPAP.
As mentioned, support of ventilation is indicated if adequate oxygen saturation cannot be maintained in spontaneously breathing patients despite 100% oxygen delivery. Assisted ventilation may also be required to correct alveolar hypoventilation despite adequate oxygen saturation. Adequacy of ventilation should be assessed clinically by chest wall expansion with further data from either ETCO2 or blood gas analysis of PCO2. Goal tidal volumes are usually 7 to 10 mL per kg, although this will vary based on lung compliance and underlying disease.
Specific ventilation strategies will vary based on underlying disease. In children with acute respiratory failure but normal lung function (e.g., CNS depression), standard airway pressures and respiratory rates are appropriate. Positive end-expiratory pressures (PEEPs) may be useful where alveolar recruitment is important to improve gas exchange (e.g., atelectasis). This can be done manually with a bag and mask, or with CPAP or BiPAP. PEEP shifts lungs to a position on the pressure–volume curve that improves alveolar ventilation by increasing the end-expiratory lung volume or functional residual capacity. Attention must be given to minimizing the risk of barotrauma. In patients with decreased lung compliance due to either stiff lungs (e.g., fibrosis) or hyperinflation (e.g., bronchiolitis or asthma), higher pressures must be used to sufficiently ventilate the child. The inspiratory:expiratory (I:E) ratio can also be tailored to the disease process. A normal or decreased I:E ratio is used in obstructive lower airway disease to extend exhalation time to better allow elimination of Co2. Increased I:E ratios of 1:5 to 1 should be utilized in alveolar or interstitial disorders to improve oxygenation. Permissive hypercapnia, accepting elevated PCO2 values as long as pH is maintained, may be advantageous, as this may allow for pressure-limited ventilation, which will reduce the risk of barotrauma.
Fluid management is another important component of care for patients with respiratory failure. In general, fluids should be titrated to maintain normal intravascular volume as determined by monitoring heart rate, blood pressure, peripheral perfusion, and urine output. However, patients with significantly increased work of breathing generate high negative intrathoracic pressure which increases venous return. When these patients transition to positive-pressure ventilation, venous return rapidly decreases and may precipitate cardiovascular collapse. Therefore, unless clinical circumstances mandate more immediate action, rapid intravascular repletion before initiating positive pressure (through noninvasive ventilation or intubation) is prudent. In contrast, for patients with interstitial disease or pulmonary capillary leak, a slightly reduced intravascular volume may improve the cardiopulmonary mechanics necessary for effective ventilation. As a result, the FIO2 requirement may be decreased and airway pressures minimized. Depending on the diagnosis, there may be other clinical indicators such as fever, vomiting, and sepsis that may affect fluid management. In severely ill or complex patients, the measurement of central venous pressure may provide a more precise guide for monitoring fluid status.
Sedation is an important adjunct to efficient assisted ventilation to reduce anxiety and increase tolerance to the presence of a tracheal tube and assisted ventilation. Morphine sulfate dosed 0.1 to 0.2 mg per kg every 1 to 2 hours or as a continuous infusion of 0.1 mg/kg/hr is often used. This is frequently combined with a benzodiazepine, such as midazolam 0.1 to 0.2 mg per kg every 1 to 2 hours or as a continuous infusion. Muscle relaxants may help optimally ventilate intubated children with severe respiratory failure, such as those with stiff lungs (e.g., severe interstitial pneumonia) or stiff chest wall (e.g., status epilepticus), by improving compliance and reducing oxygen consumption. Depolarizing agents may include repeated doses of rocuronium at 1 to 1.2 mg/kg/dose. Alternatively, vecuronium bromide can be administered starting at 0.1 mg per kg every 1 to 2 hours or as a drip at 0.1 to 0.2 mg/kg/hr.
Clinical Indications for Discharge or Admission
Patients with acute respiratory failure require hospitalization. If resuscitation efforts restore adequate oxygenation and ventilation and a stable trajectory has been established, admission to the inpatient floor for continued evaluation and management may be appropriate. However, most patients will require intensive care unit (ICU) admission, and prompt communication with the critical care team at one’s institution or an appropriate transfer facility should be an early priority in management (see Chapter 6 Interfacility Transport and Stabilization).
ASTHMA
CLINICAL PEARLS AND PITFALLS
• Standard medications for acute asthma treatment include short-acting β-agonists (SABAs), anticholinergics, and systemic corticosteroids.
• Children with severe exacerbations should receive high-dose SABA mixed with ipratropium bromide in addition to prompt systemic corticosteroids. They often require continuous SABA as well, following initial treatments.
• Intravenous (IV) magnesium sulfate should be considered for patients not improving after multiple high-dose SABA or for patients with severe exacerbations.
• Emergency physicians should consider prescribing inhaled corticosteroids (ICSs) based on the degree of asthma control.
Current Evidence
Asthma is a chronic inflammatory disease of the lower airways characterized by bronchial hyperresponsiveness and reversible bronchospasm. While there is common pathophysiology for patients with asthma, the phenotype is rather heterogeneous, likely due to many interacting factors including the level of airway inflammation, degree of bronchial hyperresponsiveness, environmental exposures, and genetic differences (which may account for 60% to 80% of interindividual variance in treatment response).
The National Asthma Education and Prevention Program (NAEPP) has published guidelines outlining diagnosis and management of acute and chronic asthma. Many institutions have implemented local acute asthma clinical guidelines, which are associated with improved quality outcomes including shorter time to medication administration, shorter length of stay (LOS), and fewer medication prescription errors.
Goals of Treatment
Acute asthma management is directed at reversing bronchospasm and treating the underlying airway inflammation. Goals of treatment include prompt administration of bronchodilators and systemic corticosteroids and identification of complications.
Clinical Considerations
Clinical Recognition
Asthma is characterized clinically by a pattern of periodic episodes of cough, wheeze, respiratory distress, and reversible bronchospasm. While wheezing is the most obvious symptom, some patients may primarily have cough without significant wheeze. Asking the family about typical symptoms for the child can provide clarification.
Asthma is generally a clinical diagnosis, and some clinicians hesitate to diagnose asthma in children younger than 24 months. However, an asthma diagnosis is appropriate if the child has compatible history suggesting the characteristic features of lower airway obstruction, bronchial hyperresponsiveness, and reversible bronchospasm. Airway inflammation levels and formal pulmonary function testing are uncommonly measured in the acute setting.
The prevalence of “lifetime” asthma (ever diagnosed) is estimated at 13% of all US children, with 6.7 million having active disease, and over 3.5 million having ≥1 exacerbation per year. Children younger than 4 years old have the highest rates of ED visits, ambulatory visits, and hospitalizations. Asthma disproportionately affects minority children, those living in urban areas, and those of lower socioeconomic status.
Triage
Prompt determination of the severity of respiratory distress will help direct appropriate therapy. Level of severity can be generally categorized as mild, moderate, severe, or impending respiratory failure. There are several validated severity scoring tools, including the Pediatric Asthma Severity Score, Modified Pulmonary Index, and Pulmonary Score. Many guidelines utilize such scores and outline severity-based therapy. These scores also allow physicians and nurses to communicate about severity and response to therapy using a standard language.
Initial Assessment/H&P
In addition to determining level of severity, obtaining asthma-specific history is helpful to inform further care and disposition. Important history includes information about the current exacerbation (duration, course, home medications administered and response to treatment, and likely trigger) as well as chronic severity of asthma (number of exacerbations during prior 12 months, number of hospitalizations, number of ICU admissions, need for intubation, use of controller therapies).
Chronic asthma severity reflects asthma control, often considered to include assessment of asthma risk (prior exacerbations requiring unscheduled visits, use of systemic corticosteroids, and hospitalizations) and impairment (asthma symptom burden and rescue medication use). While such detailed assessment may not be commonly performed in a formal manner using all of the questions outlined in the NAEPP or Asthma Control Test, clinicians can query about frequency of days or nights with increased asthma symptoms and use of albuterol. Patients with 2 or more days/nights of symptoms and/or albuterol use per week likely have chronic asthma severity in the “persistent” range and prescribing or continuing inhaled steroids is recommended.
Management
Treatment involves weight-based dosing of SABA (most commonly albuterol in the United States), anticholinergics (usually ipratropium bromide), and systemic corticosteroids (prednisone, prednisolone, methylprednisolone, or dexamethasone) (see Chapter 84 Asthma).
Inhaled SABA causes bronchodilation of airway smooth muscle through activation of β2-adrenergic receptors. Albuterol is the most commonly used SABA. It is a racemic mixture of two enantiomers—R-albuterol (binds β2-receptor and causes bronchodilation and adverse effects of tachycardia and tremor) and S-albuterol (believed to have detrimental effect on airway function). Levalbuterol contains the R-enantiomer alone, and is marketed as an alternative to racemic albuterol with fewer adverse effects (e.g., tachycardia) than racemic albuterol. However, studies are inconsistent regarding clinical superiority of levalbuterol over racemic albuterol, and the increased cost of levalbuterol must be considered. The NAEPP guidelines list levalbuterol as an option for SABA treatment at half the dose of nebulized albuterol.
Albuterol can be administered using metered-dose inhalers (MDIs) with valved holding chambers (spacers) or nebulizers. Use of both requires proper technique. While there are potential differences in lung deposition between devices, studies have found equivalency or favor MDI with spacer with regard to ED LOS and tachycardia. Although nebulizers have traditionally been the preferred devices, MDI with spacer may be considered an option for children with mild and moderate exacerbations. Studies on MDI with spacer for severe asthma exacerbations are limited. Patients with severe exacerbations have significant lower airway obstruction, which limits drug deposition in the lung, and higher overall albuterol doses using nebulizer are often necessary.
For those with mild exacerbations, it is reasonable to administer one SABA treatment and reassess need for additional therapy. Patients with moderate or severe exacerbations should receive multiple doses of SABA and anticholinergics in addition to systemic steroids.
Ipratropium bromide causes bronchodilation by blocking muscarinic cholinergic receptors. Adding anticholinergics to SABA is associated with improved pulmonary function and, importantly, has been shown to reduce hospitalization rates for those with severe exacerbations, particularly using multiple-dose protocols, and many protocols recommend its use for moderate severity as well.
Corticosteroids block formation of potent inflammatory mediators and reduce airway inflammation. Systemic corticosteroids are associated with improved pulmonary function and reduced hospitalizations. The effect on reducing hospitalizations is time dependent, maximized with early administration. A common metric regarding optimal asthma care is administration of systemic corticosteroids within 60 minutes of arrival. Systemic corticosteroids are also associated with fewer ED relapse visits and hospitalization at such return visits.
Prednisone and prednisolone are the most commonly used systemic corticosteroids, and have good oral bioavailability, tolerability, and similar effectiveness compared to IV route. Methylprednisolone or dexamethasone (IV or intramuscular) are also options favored for children in severe distress in whom oral intake may not be practical, or in those who are actively vomiting or likely to do so based on prior history. Other than these exceptions, oral corticosteroids are preferred for milder exacerbations. Dexamethasone has become more popular and a recent review observed similar outcomes and less vomiting compared to prednisone or prednisolone, though there was heterogeneity among treatment regimens.
After initial therapy, it is important to reassess the need for continued and adjunctive medications. Response to therapy can be categorized as good, incomplete, and poor. Those with good response have improvement with mild features and can be observed briefly and subsequently discharged if not requiring frequent SABA or having other indications for admission. Those with incomplete response continue to have moderate or severe features. They should receive frequent and possibly continuous albuterol, and adjunctive therapies such as magnesium sulfate heliox, or parenteral bronchodilator therapy should be considered.
Many studies have evaluated use of medications considered adjunctive (e.g., continuous albuterol, magnesium sulfate, heliox) in comparison to initial standard albuterol treatment, though, in practice, most clinicians administer them after insufficient improvement with multiple albuterol treatments. Adjunctive therapies such as magnesium sulfate and heliox can be administered in conjunction with ongoing inhaled bronchodilators, and timing may vary according to severity. Frequent reassessments during initial treatment for those with severe exacerbations, and anticipating the need for adjunctive therapy are essential to avoid delays.
Continuous nebulized albuterol treatment is recommended for patients with severe exacerbations or poor response to initial inhaled bronchodilator treatment. A systematic review found that continuous albuterol was associated with greater improvement in peak expiratory flow rate (PEFR) and lower hospitalization rate, particularly among those with moderate or severe exacerbations, with no increase in adverse effects.
Magnesium sulfate causes bronchodilation by relaxing respiratory smooth muscle. It is administered as a single IV bolus with a recommended dose of 50 to 75 mg per kg (maximum 2 g). Use of this therapy has been associated with improved pulmonary function and reduced hospitalization rates.
Heliox is a mixture of helium and oxygen, thought to improve drug delivery in obstructed airways due to its lower density and airflow resistance. The commonly used mixtures (helium:oxygen) are 70:30 or 80:20, but use in patients with significant hypoxemia may be limited. Contraindication for Heliox is pneumothorax, pneumopericardium, or pneumoperitoneum, therefore a chest radiograph (CXR) should be obtained prior to initiation.
Parenteral β-agonists are also options to consider for adjunctive therapy. Epinephrine administered intramuscularly may be an option for severe exacerbations, particularly as initial treatment for patients with significant airway obstruction when delivery of inhaled medications to the lower airways may be limited. Terbutaline may be administered subcutaneously or intravenously as a bolus and continued as an IV infusion. Although commonly included in many pediatric protocols for refractory asthma, pediatric studies regarding use are limited.
Noninvasive ventilatory support (CPAP or BiPAP) may benefit patients tiring from increased work of breathing and with impending respiratory failure. Pediatric studies are limited but suggest that it is generally well tolerated. While some studies suggest that it may reduce need for ICU admission, in practice, most patients who require ventilator support are treated in an ICU setting.
Chest radiographs (CXRs) are not routinely indicated for acute asthma exacerbations in children. Wheezing is a common symptom of asthma and pneumonia in children, therefore determining which patients warrant imaging can be challenging. Data regarding children of all ages with wheezing who had CXR, suggest that approximately 5% of febrile children will have radiographic findings consistent with pneumonia. However, the potential risks of CXR include radiation exposure and false-positive results leading to unnecessary antibiotic therapy. In general, patients with a typical asthma exacerbation do not routinely warrant imaging given this low rate of abnormal findings. In a patient with mild to moderate respiratory distress, the decision to perform a CXR may be deferred until reassessment after initial treatment; focal abnormal breath sounds may have improved suggesting atelectasis as opposed to pneumonia.
Clinical Indications for Discharge or Admission
In general, children requiring frequent albuterol (generally defined as more frequent than every 2 to 4 hours) or having persistent hypoxemia require admission. Other reasons for admission include significant dehydration, infection requiring inpatient treatment or monitoring, or medical history that may impact the respiratory system (e.g., cardiac disease, neuromuscular disorder, or metabolic disorder). Most patients requiring frequent inhaled bronchodilator therapy or adjunctive therapy (e.g., parenteral bronchodilators) will require hospitalization. Protocols regarding which therapies require an ICU setting vary by institution.
Patients discharged should be encouraged to follow up with their primary care providers (PCPs) within 1 to 3 days. Discharge instructions should include information about care following the acute visit and may include formulation of an asthma action plan. This provides an opportunity to assist patients with management during future exacerbations and to encourage partnership with PCPs for ongoing discussions and modifications of asthma care.
Inhaled steroids should be continued for patients currently taking them, and clinicians should strongly consider prescribing them from the ED when indicated. Patients with 2 or more days/nights of symptoms and/or albuterol use per week likely have chronic asthma severity in the “persistent” range and inhaled steroids are recommended. Data suggest that many patients treated for acute asthma in EDs meet criteria for persistent chronic asthma severity, yet no prescription has been provided or patients are noncompliant with therapy. Therefore, the ED visit for asthma represents an opportunity to improve outcomes for these children.
ASPIRATION PNEUMONIA
CLINICAL PEARLS AND PITFALLS
• Aspiration pneumonitis refers to chemical injury and inflammation of lung tissue after inhalation of foreign material, whereas aspiration pneumonia refers to infection of lung tissue following pneumonitis.
• Patients at risk for aspiration pneumonia include those with impaired neurologic status and gastrointestinal dysmotility.
• Initial chest radiographs may be normal following aspiration episodes.
• Treatment with antibiotics is generally reserved for patients with significant respiratory impairment and signs of infection or complicating medical history.
• Treatment with corticosteroids is not routinely indicated.
Current Evidence
Aspiration of foreign material into the lung can result in inflammation and impaired lung function. Aspiration pneumonitis refers to chemical injury and inflammation of lung tissue from inhaled foreign material, with sterile acidic gastric contents being the most common source. Aspiration pneumonia refers to infection of lung tissue following inhalation of foreign material, often due to bacteria from the oropharynx.
The pathophysiology of pulmonary disease following aspiration can be classified based on the source of the foreign material. In humans, aspirate contents with a pH lower than 2.5 are considered acidic, and such material may cause a severe chemical pneumonitis with direct injury to alveolar–capillary membranes. Effects from initial injury can occur within minutes to hours, and may include reflex airway closure and destruction of surfactant resulting in atelectasis. A granulocytic, necrotizing reaction generally follows causing exudation of fluid and protein across damaged membranes creating interstitial and alveolar edema, alveolar hemorrhage, and consolidation.
Aspirates with a pH higher than 2.5 are considered nonacidic, typically arising from aspiration of contents from the oropharynx or stomach in patients taking H2 blockers or proton-pump inhibitors. The early pathophysiologic response is similar to that seen with acidic chemical pneumonitis, with the exception of reduced alveolar neutrophilic infiltration and necrosis. The extent of lung damage from nonacid aspirates varies depending on the composition of the aspirate; clear liquid aspiration resolves quickly while sizable food particles may lead to prolonged pathologic response. Repeated aspirations occurring over an extended period may result in radiographic evidence of granuloma formation similar to that of miliary tuberculosis. Aspiration of hydrocarbons is covered separately in Chapter 110 Toxicologic Emergencies.
Goal of Treatment
Treatment of aspiration pneumonia aims at treating any underlying conditions and preventing further aspiration. Supportive measures may involve assisting ventilation and oxygenation as needed, and consideration of antibiotics.
Clinical Considerations
Clinical Recognition
Children with neurologic impairment including altered consciousness and CNS disorders that compromise normal swallowing or protective airway reflexes are at risk for aspiration. This is particularly true for chronically impaired children, although healthy children who are transiently depressed from acute neurologic deterioration, procedural sedation, or during or after seizures can also aspirate. In addition, children with decreased esophageal or intestinal motility or delayed gastric emptying are at increased risk of regurgitation of stomach contents and therefore possible aspiration. Such gastrointestinal dysmotility may be secondary to underlying disease, trauma, or medications such as opiates or those with anticholinergic properties. Similarly, anatomic narrowing or obstruction along the gastrointestinal tract can also increase the risk of aspiration.
Triage
Patients with aspiration pneumonia may present with acute severe respiratory distress, and, therefore, most will require prompt evaluation. Patients with chronic aspiration often have a more insidious course, though may have an acute event or intercurrent illness that results in more notable respiratory compromise prompting evaluation.
Initial Assessment/H&P
The reported symptoms and physical findings in patients with aspiration pneumonia are similar to patients with pulmonary infections resulting from community- or hospital-acquired bacterial or viral causes, and are further discussed in Chapter 102 Infectious Disease Emergencies.
In cases of aspiration pneumonia, a brief latent period may occur before the onset of respiratory signs and symptoms; more than 90% of patients are symptomatic within 1 hour. Fever, tachypnea, and cough are frequent findings. Hypoxia is also common, whereas apnea and shock are less likely but possible. Sputum production is usually minimal.
On examination, focal or diffuse crackles and wheezing are common. Cyanosis may appear with more severe disease. Chest radiographs (Fig. 107.1) may show either localized or diffuse infiltrates, which may be unilateral or bilateral. The chest x-ray of a patient who has aspirated may evolve from normal to complete bilateral opacification within several hours.
Management
In the acute care setting, children who aspirate require primarily supportive care. Specifically, prevention of further aspiration by gastric decompression, oropharyngeal suctioning, and proper positioning should be a goal. Supplemental oxygen should be administered as needed. Endotracheal intubation is indicated if airway reflexes are acutely compromised or for severe cases with impending respiratory failure. Children with impaired baseline pulmonary function may require significant supportive care after aspiration.
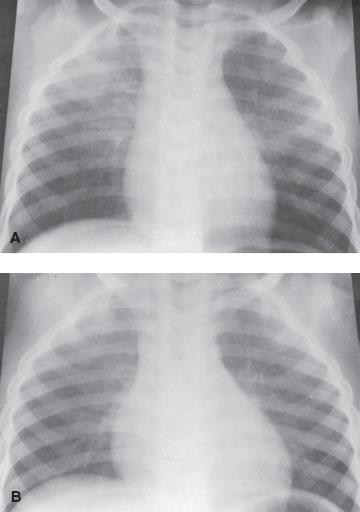
FIGURE 107.1 A: Blood aspiration. A 3-year-old boy with tachypnea 1 day after surgery for enlarged adenoids/tonsils. Chest film shows an infiltrate in the right upper lobe and left lower lobe. B: The chest film 2 days later shows clearing of the infiltrate in the right upper lobe and left lower lobe.
The suspicion of aspiration should be confirmed with a chest radiograph. Some children who aspirate may have relatively normal radiographs early in the course but significant symptomatology and findings. Conversely, radiographs may be significantly abnormal in the face of minimal clinical symptoms in patients with aspiration of hydrocarbon or other volatile agents (further discussed in Chapter 110 Toxicologic Emergencies).
The decision to initiate antibiotic therapy is challenging. Most physicians agree that infection plays little role in the initial pulmonary complications after aspiration, that is, aspiration pneumonitis. However, pathogenic bacteria from the oropharynx may accompany foreign material, resulting in direct inoculation of lung tissue. Alternatively, following acid aspiration, the injured lung is vulnerable and secondary bacterial infection may occur in up to half of these cases. There is no strong data to suggest that prophylactic antibiotic therapy will prevent subsequent infection in a patient with chemical pneumonitis. Moreover, fever, purulent sputum, leukocytosis, and pulmonary infiltrates may result from chemical inflammation alone, making the distinction between aspiration pneumonitis and aspiration pneumonia difficult. A reasonable initial approach is to defer antibiotic treatment in favor of careful observation in a well-appearing child and empirically treat only those with tenuous respiratory status or compelling clinical evidence of infection, or significant medical history that may complicate their clinical course.
For those who develop infection, two distinct patterns are possible. A localized necrotizing bacterial pneumonia, abscess, or empyema may result from a heavily infected aspirate. Although opinions vary, anaerobic organisms, either alone or as polymicrobial infection with aerobes, are likely etiologies in such cases. The second pattern of infection is that which follows large aspirates, usually of the acidic type. Aerobic rather than anaerobic organisms predominate in this case; gram-negative organisms, such as Pseudomonas aeruginosa, and gram-positive organisms, such as staphylococci, may be isolated.
The choice of antibiotics can be guided by the clinical setting and the results of properly obtained specimens for culture. Size and type of aspirate are considerations as are settings in which the aspiration occurs. Aspiration pneumonias developing outside the hospital generally involve aerobes and are adequately treated with ampicillin or clindamycin, whereas nosocomial infections require broader aerobic and anaerobic coverage. Clindamycin with gentamicin, or ampicillin/sulbactam, is often used. In neurologically impaired children, with either aspiration or tracheostomy-associated pneumonia, antibiotics effective against penicillin-resistant anaerobic bacteria and P. aeruginosa have been shown to produce superior clinical and microbiologic responses.
The use of corticosteroids in the treatment of aspiration pneumonia is controversial. Because experimental evidence indicates minimal benefit at best and because the concomitant immune suppression may contribute the development of secondary bacterial pneumonia, their administration is not usually indicated in the ED.
Clinical Indications for Discharge or Admission
Children with significant aspiration pneumonia, diagnosed either by clinical suspicion or radiograph, require admission to the hospital.
BRONCHOPULMONARY DYSPLASIA
CLINICAL PEARLS AND PITFALLS
• Diagnosis is usually established prior to presentation to the ED.
• Management involves supportive measures, including supplemental oxygen, assurance of adequate hydration, and often bronchodilators.
Current Evidence
BPD is a chronic respiratory disease, usually occurring in premature infants. BPD is a clinical diagnosis, defined as requiring supplemental oxygen at a prescribed postconceptual or chronologic age, with associated radiographic findings. The specifics of the diagnostic parameters have changed over time.
The etiology of BPD is thought to be multifactorial. While newer data suggest a genetic predisposition, previously defined risk factors include prematurity, relatively long duration of supplemental oxygen therapy after birth, requiring positive-pressure ventilation, and inadequate nutrition. The disease process is thought to begin after inflammation and injury to the lung, with resultant arrest of alveolar septation and impaired microvascular development. This occurs most commonly in infants with hyaline membrane disease or other acute perinatal lung disease. Infants with apnea, congenital heart disease, or other illnesses requiring prolonged ventilation in the first weeks of life are also at risk. Utilization of improved ventilation strategies, as well as antenatal glucocorticoids, surfactant, and improvement in nutrition, has improved outcomes in children with BPD.
In the emergency setting, patients with BPD may present with acute exacerbations of their chronic lung disease. Furthermore, BPD is a risk factor for increased severity of other respiratory illnesses.
Goals of Treatment
Treatment of BPD exacerbations involves supportive care with attention to need for supplemental oxygenation and ventilation support, as well as hydration status. Bronchodilators, ICSs, and diuretics may also be helpful.
Clinical Considerations
Clinical Recognition
BPD should be suspected among premature infants, as well as in children previously requiring either assisted ventilation and/or prolonged supplemental oxygen therapy.
Emergency physicians most often will evaluate children with BPD when their underlying disease is worsened by intercurrent acute respiratory infections. More than 50% of infants with BPD require admission for respiratory illness within a year of their diagnosis. Of particular importance is respiratory syncytial virus (RSV) infection, which typically causes bronchiolitis with fever, tachypnea, crackles, and wheezing. Patients with BPD who develop RSV bronchiolitis are prone to more severe courses, including higher rates of ICU admission, need for mechanical ventilation, and mortality.
Triage
Children with BPD exacerbations may present with significant distress and may require prompt evaluation and treatment.
Initial Assessment/H&P
Signs and symptoms of BPD exacerbations vary based on severity of the underlying disease; therefore, recognizing interim worsening of disease requires an understanding of baseline examination findings and pulmonary function.
Children with BPD are typically tachypneic at baseline, with some degree of retractions that worsen with even mild respiratory or febrile illnesses. Findings on auscultation including crackles, wheezes, or decreased breath sounds may be present at baseline and worsened with exacerbations or acute illness. Infants with BPD may have a history of failure to thrive, often resulting from concomitant nutritional issues, or from increased energy expenditure secondary to chronic increased work of breathing. Chest radiographs (Fig. 107.2) often demonstrate varying amounts of hyperinflation; several patterns occur, including cystic areas with signs of fibrosis, which are often confused with congenital lobar emphysema or severe CF. Comparison with prior CXRs is important to distinguish old changes from new infiltrates.
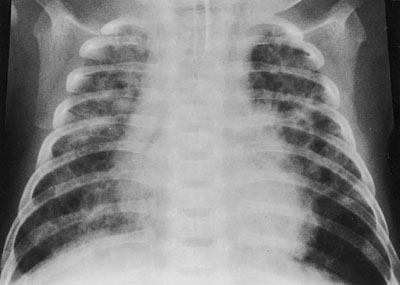
FIGURE 107.2 Bronchopulmonary dysplasia. This 2-month-old child was treated with mechanical ventilation during the first days of life for hyaline membrane disease. The chest film shows generalized overaeration and coarse nodularity with multiple cyst-like areas throughout both lung fields.
Management
Management of children with BPD and intercurrent respiratory illnesses is primarily limited to supportive care. If the exacerbation is mild, outpatient therapy may be indicated with frequent follow-up every 1 to 2 days. However, for infants with moderate to severe BPD at baseline, even mild deterioration may herald early respiratory failure. Ensuring hydration by oral or IV routes, addressing hypoxemia, and, when necessary, providing assisted ventilation for hypercarbia and respiratory acidosis are the mainstays of therapy.
Pulse oximetry is important to assess for hypoxemia. ETCO2 measurement through noninvasive means or PCO2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







