Pulmonary Embolism
Prathap Sooriyakumaran and Jeffrey A. Tabas
Pulmonary embolism (PE) is a relatively common, life-threatening condition that presents a diagnostic dilemma for the emergency practitioner. The incidence in the United States is estimated at 650,000 to 900,000 patients per year, resulting in as many as 200,000 deaths per year (1). This makes PE the third leading cause of cardiovascular death. Correct diagnosis and treatment significantly decreases morbidity and mortality (2). Accurate diagnosis is essential, as a missed or delayed diagnosis is associated with increased rates of adverse outcomes (3), and over diagnosis subjects a patient to the risks of unnecessary anticoagulation as well as to the psychological and financial implications of carrying such a diagnostic label.
CLINICAL PRESENTATION
Pulmonary emboli may result from a myriad of causes. Most commonly, emboli arise from thrombi in the legs, but they may also originate from anywhere in the venous system proximal to the pulmonary arteries, such as pelvic veins, nonportal abdominal veins, upper-extremity veins, or right-sided heart valves. Other sources include fat emboli, usually in association with long-bone fractures, amniotic fluid emboli (in the setting of labor and delivery), air emboli, and septic emboli.
The signs and symptoms of pulmonary emboli result from the effects of obstructed pulmonary vasculature. This may result in local tissue ischemia or infarct, increased ventilation–perfusion mismatch that can cause increased alveolar dead space or increased alveolar–arterial oxygen gradient, and increased pulmonary vascular resistance that can cause right heart strain or failure. The spectrum of presentation varies from asymptomatic patients to those with mild subjective complaints to hemodynamically unstable patients with severe hypotension, respiratory failure, or pulseless electrical activity. The terms “submassive” and “massive” PE both describe symptomatic PE, with a systolic blood pressure less than 90 typically used to distinguish “massive” hemodynamically unstable PE, from “submassive” hemodynamically stable PE that nonetheless has some degree of right ventricular dysfunction and increased pulmonary hypertension. Differences in size, quantity, and location of emboli as well as underlying patient characteristics (such as cardiopulmonary reserve) contribute to the wide spectrum of presentation.
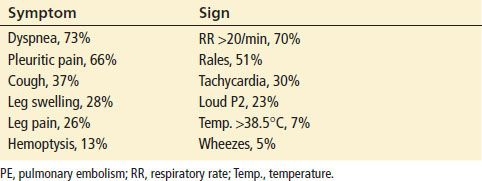
The most common signs and symptoms associated with the presence of PE are listed in Table 79.1 (4). A prospective cohort study of 7,940 patients with diagnosed PE found that patient history of PE or deep vein thrombosis (DVT), unilateral leg swelling, surgery within the previous 4 weeks, current estrogen use and hypoxemia (saturation <95%) were the strongest predictors of PE, with odd ratios (OR) between 2 and 3 (5). Common symptoms investigated include dyspnea (OR 1.26), pleuritic chest pain (OR 1.53), and symptoms of DVT such as unilateral leg swelling (OR 2.60). Although low-grade fever is not infrequent, temperatures >38.5°C are less common and suggest other causes (temperature >38.5°C had an OR of 1.13, but this was not statistically significant). Unfortunately, many of these signs and symptoms are manifestations of other conditions, and there is no combination of clinical findings that has been shown to be diagnostic for PE. In addition, the “classic” triad of hemoptysis, pleuritic chest pain, and tachypnea is uncommon.
TABLE 79.1
Relative Frequency of Presenting Symptoms and Signs in Patients with PE
Classic risk factors (Table 79.2) for thrombosis include Virchow’s triad of hypercoagulability, venous stasis, and endothelial injury. Commonly acquired hypercoagulable states include cancer, pregnancy, and estrogen therapy. There is increasing understanding and discovery of heritable thrombophilias, but these risk factors are often unknown at the time of presentation. Venous stasis may result from prolonged immobilization, recent surgery, extended travel, or medical conditions such as paraplegia or previous stroke. Damage to the vascular endothelium may result from fractures, surgery, catheter placement, or other forms of trauma. Demographic factors, such as advanced age, should also be taken into consideration. However, up to 30% of patients with a diagnosed PE have no known risk factors. In summary, although the presence of risk factors increases suspicion for PE, lack of known risk factors does not exclude the diagnosis.
TABLE 79.2
Risk Factors for PE

DIFFERENTIAL DIAGNOSIS
Given the wide spectrum of signs and symptoms associated with PE, the differential diagnosis in most presentations is extensive. Other causes of dyspnea or chest pain include pulmonary and cardiac causes such as chronic obstructive pulmonary disease (COPD) or asthma, congestive heart failure (CHF), pulmonary infections, pericarditis, acute coronary syndrome, aortic dissection, pneumothorax, trauma, or musculoskeletal pain. Abdominal sources such as biliary colic or pancreatitis may mimic a PE. The evaluation can be complicated by the fact that conditions such as CHF can both mimic signs and symptoms of PE as well as be a risk factor for PE. Musculoskeletal pain and anxiety are always diagnoses of exclusion and should be made only after more life-threatening causes have been confidently eliminated.
ED EVALUATION
Initial management of PE consists of rapid assessment and stabilization for immediate life-threatening conditions. When appropriate, intravenous access should be established, and cardiac and pulse oximetry monitors should be placed. Laboratory testing, electrocardiography, and chest radiography should be expedited. The probability of disease (see subsequent text) should be determined based on the patient’s signs, symptoms, identifiable risk factors, and the presence of an acceptable alternate diagnosis.
A complete blood count and serum chemistry panel are usually indicated in the general assessment of any patient with chest pain or shortness of breath. A creatinine level is helpful if contrast administration is planned. All patients suspected of having a PE should undergo an electrocardiogram (ECG). The ECG is a nonspecific test for PE, and the primary purpose is to detect other disease processes such as pericarditis or acute coronary syndromes. The most common ECG findings in patients with PE are nonspecific ST and T-wave abnormalities. Other common findings include tachycardia and T-wave inversion in the anterior precordial leads (V1 to V4). The classic ECG finding of S1Q3T3, consisting of an S wave in lead 1, and a Q wave with a flipped T wave in lead 3, is neither sensitive nor specific for PE. In a secondary analysis of a prospective, observational cohort of 6,049 ED patients who were tested for PE and had an ECG, both the findings of S1Q3T3 and T-wave inversions in V1 to V4 had positive likelihood ratios (LR+) of 3.7 and tachycardia had a LR+ of 1.8 (6). These findings were infrequent overall, but were observed more frequently in patients with the final diagnosis of PE compared to patients who did not have PE.
A chest radiograph should be obtained for patients with suspected PE. Given the lack of specific findings for PE, the primary purpose is again to detect other disease processes, such as a pneumothorax, pulmonary edema, malignancy, or pneumonia. In one large study of patients with proven PE, the most common chest radiographic interpretations were cardiac enlargement (27%), normal (24%), pleural effusion (23%), elevated hemidiaphragm (20%), pulmonary artery enlargement (19%), atelectasis (18%), and parenchymal pulmonary infiltrates (17%). Cardiomegaly is a common associated finding with PE, given that CHF is a risk factor. Classic radiographic findings, which occur infrequently, include Westermark sign (a prominent central pulmonary artery with decreased pulmonary vasculature distally) and Hampton’s hump (a wedge-shaped, pleural-based opacity representing pulmonary infarct) (7).
Arterial blood gases are rarely helpful in the evaluation of PE. One analysis of more than 100 patients with PE who lacked prior cardiopulmonary disease revealed that 38% had a normal alveolar–arterial (A–a) gradient. Patients with prior cardiopulmonary disease who proved not to have PE, had an elevated A–a gradient 24% of the time (8). One potentially useful scenario may occur when a patient is found to have a large, unexplained A–a gradient. However, these authors rarely obtain arterial blood gases for suspected PE alone.
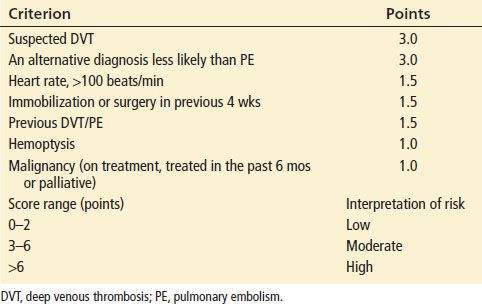
Based upon the history, physical examination, and preliminary laboratory evaluation, the clinician should categorize a patient into very low (<2%), low (2% to 15%), moderate (15% to 40%), or high probability (>40%) suspicion for PE. Several clinical decision rules have been developed in an attempt to improve implicit clinician judgment, however, they appear to be no better than gestalt assessment by experienced providers in predicting probability of PE (9,10). These rules appear to be most effective at improving risk stratification for the clinically inexperienced practitioner and to provide some uniformity of approach within a department. The Wells Criteria (Table 79.3) is the most extensively tested decision rule. A modified approach, in which patients with a score less than or equal to 4 are considered safe for D-dimer testing and those greater than 4 are not, has been shown to be safe and effective (11).
TABLE 79.3
Criteria of Wells et al. for Assessment of Pretest Probability for PE
Evaluation for PE should be based on pretest probability. See Figure 79.1 for an algorithm summarizing our recommendations for how to proceed with the evaluation for PE. The results of diagnostic testing should be used to determine sequentially what further evaluation is needed to exclude or confirm the presence of PE. A posttest probability of 1% to 2% is a generally accepted risk level. This would presumably lead to a subsequent complication rate of <0.7%. Some patients and physicians may be willing to accept more or less risk.
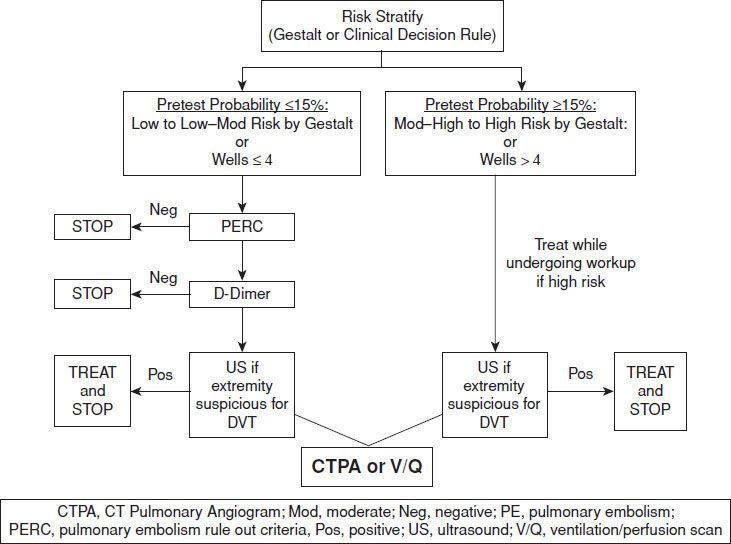
FIGURE 79.1 Workup of PE in the ED.
For patients who are low to low-moderate probability (less than 15%) stratified either by clinical decision rule or gestalt, Kline et al. (12) have demonstrated that patients with a zero score on the Pulmonary Embolism Rule Out Criteria (PERC) do not need further evaluation for PE. PERC consists of 8 criteria (Table 79.4). When none of these are present in patients with low to low-moderate pretest probability, the risk of PE or death is similar to that of patients with negative V/Q or CT pulmonary angiography. In a meta-analysis of 11 studies investigating the diagnostic performance of PERC for PE, the pooled sensitivity and specificity was 0.97 (95% confidence interval 0.96 to 0.98) and 0.23 (95% confidence interval 0.22 to 0.24), respectively (13). For patients with less than 15% pretest probability who are PERC positive, the next step in evaluation is a D-dimer test. D-dimers are degradation products of cross-linked fibrin and are therefore elevated when there is activation of the coagulation pathway, as occurs in the setting of PE. Because of their nonspecific nature, D-dimers are also elevated in other conditions such as cancer, pregnancy, recent trauma, and even CHF. When used appropriately, a sensitive D-dimer assay is an effective screening test for PE, eliminating the need for further radiologic imaging. Most current assays show sensitivities as high as 90% to 95%. Bayesian analysis reveals that in a patient with 15% pretest probability (the upper limit of “low probability”), a negative likelihood ratio of 0.12 or less is required to provide a posttest probability of <2%. Most of the rapid, sensitive D-dimer tests demonstrate negative likelihood ratios within this range. Therefore, a negative result using currently available rapid, sensitive D-dimer assays is adequate to exclude PE in a patient with low to low-moderate pretest probability (14,15). Note that a positive D-dimer result has little impact on posttest probability.
TABLE 79.4
Pulmonary Embolism Rule-Out Criteria (PERC)