Joanne Sandberg-Cook
Proteinuria and Hematuria
Proteinuria and hematuria are relatively common findings on routine urinalysis. However, these findings can also be signs of serious disease or neoplasm. For example, research demonstrates that there is a positive linear association between the magnitude of proteinuria and an increase in the risk of cardiovascular disease and end-stage renal disease (ESRD).1 Hematuria is the most common sign of bladder cancer. Because of the potential seriousness of these findings, careful, systematic evaluation is essential.
Proteinuria
Definition and Epidemiology
Approximately 15 kg of protein is filtered through the adult kidney each day, with normally less than 150 mg excreted.2 Proteinuria, generally defined as urinary protein excretion of more than 150 mg/day (10 to 20 mg/dL), is the hallmark of renal disease. Microalbuminuria is defined as the excretion of 30 to 150 mg of protein per day and is a sign of early renal disease, particularly in patients with diabetes.3 Macroalbuminuria is occasionally used to describe rates of more than 300 mg/day.
Proteinuria can be classified as transient or persistent. Transient proteinuria is caused by a temporary change in glomerular hemodynamics, which causes the excess of protein. These conditions are usually of a benign or self-limited nature and include orthostatic (postural) proteinuria, dehydration, fever, exercise, and emotional stress. Congestive heart failure and seizures can also cause transient proteinuria.4 Persistent proteinuria is defined as 1+ protein on a standard dipstick (which corresponds to approximately 30 mg/dL) two or more times during a 3-month period.4 Persistent proteinuria indicates a pathologic process, and the etiology must be investigated. Some common causes of persistent proteinuria are listed in Box 148-1.
Although isolated proteinuria is not necessarily associated with excess morbidity and mortality, it can be a sign of serious systemic disease. Evidence suggests that changes in low levels of proteinuria are predictive of the annual decline in glomerular filtration rate and the development of ESRD in persons with nondiabetic kidney disease.1 Even a slight increase in proteinuria has been shown to be an independent risk factor for ESRD. Therefore, asymptomatic proteinuria warrants further evaluation.
In the United States, diabetes is the leading cause of ESRD, and in both type 1 and type 2 diabetes, microalbuminuria is the first sign of deteriorating renal function (see Chapter 206). As kidney function declines, microalbuminuria becomes full-fledged proteinuria. Hypertension is the second leading cause of ESRD. ESRD has a high annual mortality worldwide, with millions having no access to treatment.
Urinary albumin excretion has also been shown to predict blood pressure progression in nondiabetic, nonhypertensive individuals and appears to precede progression to higher blood pressure stages.5 Therefore, proteinuria may be a useful biomarker for identification of individuals who are at risk for development of hypertension. In addition, persistent proteinuria in excess of 1 g/day has been associated with increased cardiac morbidity and mortality, especially heart failure.6
Certain population groups, including African Americans, Native Americans, Hispanic Americans, and Pacific Islanders, are at increased risk for development of proteinuria. Aging and obesity are also risk factors for development of proteinuria.7
Pathophysiology
Protein excretion is affected by three factors: (1) prevention of excretion by the glomerular capillary wall, (2) reabsorption and catabolism by the proximal tubule cells, and (3) production of low-molecular-weight proteins.3 Therefore, proteinuria is classified as glomerular, tubular, or overflow in origin. Glomerular proteinuria is the most common type of persistent proteinuria, and albumin is the primary urinary protein.3 Tubular proteinuria results when malfunctioning tubule cells no longer metabolize or reabsorb the protein that has been normally filtered. In this condition, low-molecular-weight proteins are the predominant type of protein, and the amount rarely exceeds 2 g/day. Overflow proteinuria occurs when low-molecular-weight proteins overwhelm the ability of the tubules to reabsorb filtered proteins.2
Clinical Presentation
The clinical presentation of the patient with proteinuria can vary from healthy young adults with functional proteinuria related to prolonged exercise to seriously ill diabetic patients with nephrotic syndrome. All individuals should therefore be screened for proteinuria by routine dipstick testing. Especially important is the routine screening of pregnant women. Proteinuria before 24 weeks’ gestation indicates a likely glomerulonephritis, whereas proteinuria after 24 weeks’ gestation is usually a sign of preeclampsia.8
Persistent proteinuria in patients with diabetes is usually a result of diabetic nephropathy. However, uncontrolled diabetes mellitus may cause transient proteinuria, most likely as a result of hyperfiltration and decreased tubular reabsorption.9
Physical Examination
With proteinuria, a complete and thorough history is essential. Specific areas of focus should include recent acute or chronic illness, surgery, diagnostic procedures (especially those requiring contrast media), urinary frequency or symptoms suggesting infection, risk factors for human immunodeficiency virus (HIV) infection, medications taken (including over-the-counter medications), family history of renal disease or diabetes, and recent physical activity (especially exercise or cold weather activities). The physical examination should be comprehensive and thorough; in the case of coexistent diabetes, the severity of the diabetes should be assessed to determine whether it correlates with the severity of proteinuria. Diabetic retinopathy is often present in patients with diabetic renal disease.10
Diagnostics and Differential Diagnosis
Proteinuria is usually detected on routine dipstick testing, and any value of 1 or greater on two or more occasions should be investigated. Limitations of dipstick testing include false-negative results caused by dilution, inability to detect microalbuminuria (although ultrasensitive dipstick tests are now available that can measure low rates of microalbuminuria), false-positive results caused by certain medications, and inability of dipstick reagents to detect light-chain proteins.4
Once proteinuria has been identified, unless the cause is readily apparent (e.g., preeclampsia or diabetes), the urine should be tested for Bence Jones proteins (the presence of which suggests multiple myeloma). In addition, a full blood chemistry panel with fasting blood glucose concentration, hemoglobin A1c (HbA1c), lipid profile, urine culture and sensitivity, and complete blood count (CBC) with differential are indicated. Further evaluation of persistent proteinuria usually includes determination of 24-hour urinary protein excretion or spot urinary protein/creatinine ratio, microscopic examination of urinary sediment, urinary protein electrophoresis, and additional assessment of renal function.4 A diagnostic flowchart for the evaluation of proteinuria is provided in Figure 148-1.
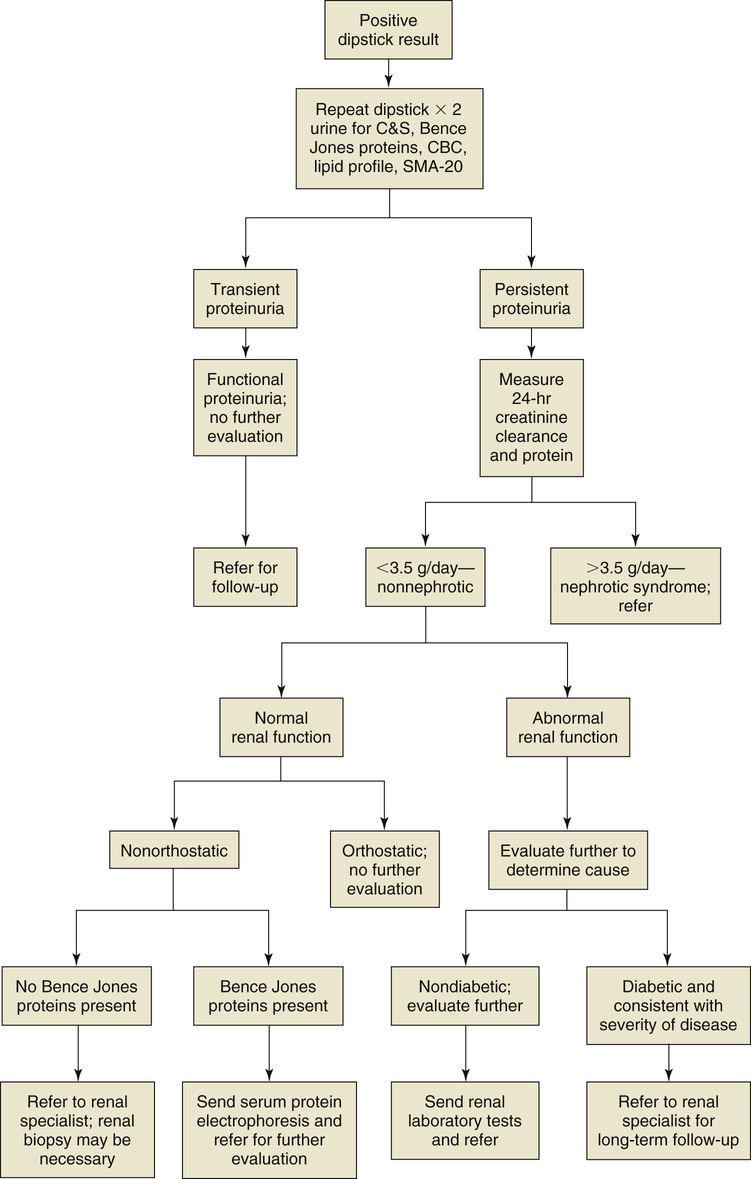
It is important to determine whether the proteinuria is persistent or transient. Transient proteinuria in an otherwise healthy patient that is secondary to an identifiable cause (e.g., exercise, fever, congestive heart failure) may be classified as functional proteinuria and does not require further diagnostic testing or evaluation.
Persistent proteinuria that cannot be classified as functional proteinuria requires further investigation. Investigation should begin with a 24-hour measurement of urine protein and creatinine clearance to determine the urinary protein excretion and the protein/creatinine ratio.11 Although this has been the gold standard, it is complicated for patients and errors are common. If the excretion rate is 3.5 g/day or more, the patient by definition has nephrotic syndrome,11 which is usually accompanied by hypoalbuminemia, hyperlipidemia, and edema. Nephrotic syndrome mandates a nephrologist’s evaluation. Diabetes is the leading cause of nephrotic syndrome and accounts for 75% of all cases.11
If the 24-hour urinary protein excretion rate is less than 3.5 g/day, renal function should be classified as normal or abnormal. Proteinuria in the presence of normal renal function is defined as “isolated” proteinuria; in these patients, the next step is to determine whether the proteinuria is orthostatic or nonorthostatic.11 Urinary protein excretion can increase after prolonged standing, and therefore three early morning voids should be checked for protein. If all the results are negative, a diagnosis of orthostatic proteinuria can be made, and no further diagnostic tests are necessary.11 However, referral to a renal specialist is also appropriate because this is a poorly understood although generally benign and self-limited condition.
An alternative to 24-hour urine testing for total protein is the measurement of the urine protein/creatinine ratio using a single urine specimen and a single blood draw. The ratio is the same as the amount of grams excreted daily, so a ratio of 3.5 is equal to 3.5 g of protein per day excreted.
Patients with nonorthostatic proteinuria and normal renal function and without an elevation in Bence Jones proteins should be referred to a renal specialist. A renal biopsy may be needed to determine the cause of the proteinuria. The presence of Bence Jones proteins warrants serum protein electrophoresis and a referral for further evaluation to exclude multiple myeloma.
Other diagnostic tests depend on presentation and differential diagnosis. Collagen disease, glomerulonephritis, hepatitis-induced vasculitis, urate-related renal disease, diabetes, and other systemic disease or structural abnormalities should be considered in the evaluation of proteinuria.11





