PROCEDURAL SEDATION
JEANNINE DEL PIZZO, MD, JOEL A. FEIN, MD, MPH, AND STEVEN M. SELBST, MD
Injuries and painful medical conditions are common among children presenting to the emergency department (ED). Some medical conditions may require a procedure which itself may be painful or may require a child to be absolutely still. ED physicians are obligated to manage pain appropriately and safely, and ensure that procedures are performed under conditions that limit harm to the patient. There have been great advances in the recognition, assessment, and management of pain. As a result, the care of infants and children who undergo painful procedures in the ED has improved considerably. Performing procedural sedation and analgesia (PSA), when indicated, is a necessary skill for the ED physician, and is essential to providing comfort and quality care to children and their families.
BACKGROUND
Barriers to Treatment of Pain in Children
Historically, pain in children has been underrecognized and undertreated. While improvements have been made, pediatric pain is still not always addressed satisfactorily in the ED and the reason is multifaceted. Adult patients who clearly indicate that they are in pain generally get a direct response from a physician, whereas a young child who is crying or whimpering may not. Crying or whimpering may be developmentally normal in the absence of pain and may reflect hunger, fatigue, anxiety, or displeasure, for example. Additionally, young children cannot describe or localize their pain, therefore, it is sometimes ignored or presumed not to exist. Some ED physicians avoid giving adequate analgesics to children because they are unfamiliar with medication options in the pediatric population, are uncomfortable with side effects, or mistakenly fear it will lead to drug addiction. Untoward side effects, such as hypotension and respiratory depression are commonly feared consequences of narcotic use in children. Although these fears may be legitimate, respiratory depression and hypotension are unlikely to occur if proper protocols are adhered to, and should be manageable in the ED in the event they do occur. Racial, ethnic, and cultural factors also influence pain and its management in the ED. There is evidence that for long-bone fractures and abdominal pain, children of Black and Hispanic background are less likely to receive analgesia than White children. Furthermore, in a busy ED, physicians are often forced to concentrate on other aspects of resuscitation and care before managing pain. Plans for pain control, therefore, may be forgotten because of other priorities. Some physicians avoid analgesics because they do not want to mask symptoms. Topical anesthetics may be avoided because it is inconvenient to wait for them to take effect. This can delay care, so some physicians may underuse analgesics and convince a young child that a painful procedure or repositioning of an extremity will hurt only for a minute. Forceful restraint (instead of medication) is then used, and more pain is inflicted on an already uncomfortable child.
Impact of Pain and Importance of Successful Pain Management
Emergency physicians must understand that pain is an individual experience and many factors contribute to the degree of pain that a child experiences for any given condition. Children of all ages can experience pain; it is believed that even neonates by 26 weeks’ gestation respond to tissue injury with specific behavior and with autonomic, hormonal, and metabolic signs of distress. Newborns feel pain and react to painful stimuli (e.g., circumcision) with wiggling motions and crying. Young children often have an exaggerated fear of needles, while older children may be better able to understand the need for a painful procedure; they are usually less anxious and better able to tolerate the inflicted pain. However, an older child may have a better understanding of the significance of an injury or an illness that could cause depression, anxiety, and more pain. Similarly, parental response (anxiety or reassuring calm) may affect a child’s perception of pain. Caregivers can experience elevated heart rate, blood pressure, and anxiety during painful procedures. Not surprisingly, parental distress–promoting behaviors may increase childhood distress. Other psychological factors, such as the child’s emotional state, personality traits, gender, or cultural background, may impact their anxiety, and this can also alter the degree of pain. Some children seem to have a hypersensitivity to pain, whereas others tolerate it well. Certain genotypes, such as the CYP2D6 polymorphisms and opioid receptor OPRM1, can mediate the metabolism and efficacy of certain opiate medications, while the COMT V158M variant has been associated with decreased need for pain medication. The context of an injury also plays a role; children who are hit during play may not complain of pain, yet may experience pain if the injury was meant as an attack or as punishment. A child’s past experience with painful stimuli is also meaningful. One study showed that inadequate analgesia for one painful procedure might diminish the effect of adequate analgesia in subsequent procedures. Of course, the painful stimulus itself is important, and a stimulus that causes a great deal of tissue damage may hurt more than one that causes minor injury. Figure 140.1 summarizes the components of a pediatric patient’s pain experience.
Uncontrolled pain may lead to hyperalgesia, a state where the painful stimulus causes more pain than normally expected. Infants who undergo a painful experience develop an altered response to future episodes of pain. For instance, infants circumcised without anesthesia show increased distress during routine immunizations at 4 to 6 months of age, compared with those who received topical local anesthetic at the time of circumcision. This has also been demonstrated in neonates who had repeated heel sticks. Furthermore, surveys of patients, parents, and families show that satisfaction with the ED experience is highly dependent on the degree of pain a patient experiences and the efforts made to alleviate the pain. One study demonstrated that more than 75% of caregivers would be willing to pay $15 and more than one-third would pay $100 to make an intravenous (IV) cannulation procedure painless. The Joint Commission has specific guidelines stating that patients have the right to pain assessment and treatment. This pain must be frequently reassessed, and the pain must be appropriately addressed with adequate analgesia.
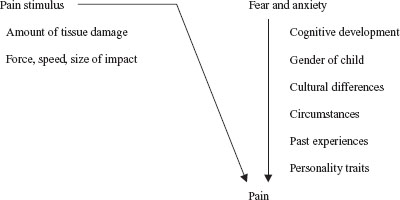
FIGURE 140.1 Components of pain. (Adapted from Schecter NL. Pain and pain control in children. Curr Probl Pediatr Adolesc Health Care 1985;15:4–67.)
Realistically, pain in the pediatric patient who presents to the ED may never be eliminated completely. Efforts must be made, however, to relieve pain as much as possible and strive toward the “ouchless” ED (Table 140.1).
Assessment of Pain in Children
In 2001, the Joint Commission standards on pain management went into effect including screening for pain and periodic reassessment. The clinical evaluation of pain may be accomplished through physiologic measurements, behavioral assessment, or self-report. Infants and preschool children (younger than 5 years of age) cannot understand the nature of self-report scales, and their assessment, therefore, relies on observer report. Physiologic indicators of pain include heart and respiratory rates, blood pressure, and palm sweating. Of interest, but not yet of practical use in the emergency setting, is the correlation between an individual’s pain and the acutely measured levels of stress hormones, such as cortisol, catecholamines, and glucagon.
TABLE 140.1
POSSIBLE REASONS FOR INADEQUATE PAIN CONTROL IN EMERGENCY DEPARTMENT

Observational behavioral pain assessments can, however, measure markers of behavioral distress experienced by the child and noted by caregivers. These include facial expression, verbal expression, and positioning. Examples of behavioral pain scales are the Behavioral Pain Score and the Children’s Hospital of Eastern Ontario Pain Scale. Some observational pain scales use a combination of behavioral and physiologic measurements. Examples of such combination scales unique to neonatal population are the Neonatal Infant Pain Scale, Neonatal Pain Agitation and Sedation Scale, and the Pain Assessment Tool.
Self-report pain scales are the best indicators of pain and are the reference standard for assessing pain in children. Standard self-report assessment tools, such as visual analog scales (VASs), are more reliable indicators of pain when completed by the patient rather than by observers. Young children (between ages 4 and 7 years) can reliably use picture scales with faces in different phases of happiness and crying. The Wong–Baker FACES Pain Rating Scale (Fig. 140.2) is one example of this type of ordinal scale. For older children and adults, a VAS consists of a 10-cm (100-mm) horizontal line with end points marked as “no pain” (0) to “worst possible pain” (10). The VAS has been further enhanced for children by allowing them to use multiple modalities for pain rating such as allowing the child to determine changes in height, thickness, and color as the pain intensity increases, as well as capitalize on the child’s ability to discriminate his or her pain using at least one of these dimensions.
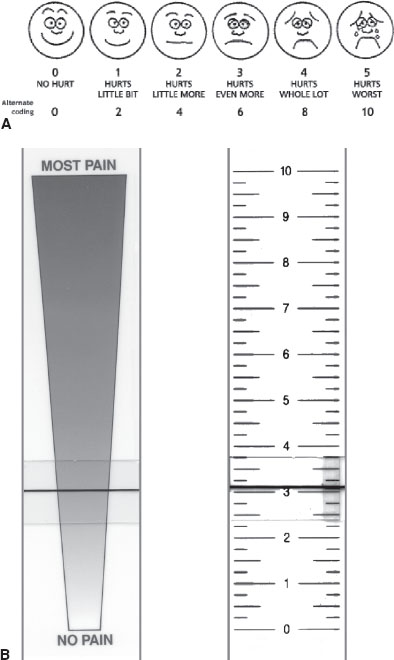
FIGURE 140.2 A: The Wong–Baker FACES Pain Rating Scale. (From Wong DL, Hockenberry-Eaton M, Wilson D, et al. Wong’s essentials of pediatric nursing. 6th ed. St. Louis, MO: Mosby, 2001:1301. Copyright Mosby, Inc. Reprinted by permission.) B: The front and back of a visual analog scale.
TABLE 140.2
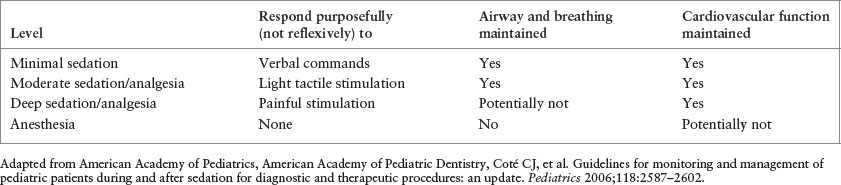
DEFINITIONS OF FOUR LEVELS OF SEDATION AND ANESTHESIA
PROCEDURAL SEDATION AND ANALGESIA
Definitions
When discussing PSA, it is useful to review terminology which is most accurately categorized by the intended effects, rather than by the specific medications used. Analgesia refers to an agent that reduces or eliminates pain in response to a normally painful stimulus. Sedation refers to a state of drug-induced depressed level of consciousness. Certain medications may have one or both of these properties and are used to that effect. For example, topical lidocaine for a laceration repair is an analgesic, but will not affect consciousness. Midazolam causes an alteration in level of consciousness, but does not take away pain. Ketamine is an example of a medication that has both analgesic and sedative properties.
PSA has replaced conscious sedation in the lexicon of emergency medical treatment of ill or injured patients. The American College of Emergency Physicians (ACEP) defines PSA as “techniques of administering sedatives or dissociative agents with or without analgesia to induce a state that allows the patient to tolerate unpleasant procedures while maintaining cardiorespiratory function.” The now outdated term conscious sedation is no longer acceptable as it does not allow for describing the depth of sedation desired or achieved. The Joint Commission accepts the continuum of depth of sedation proposed by the American Society of Anesthesiologists (ASA) in 1999. It is important to focus on the word continuum as it highlights the essential point that a patient can easily move along the continuum from one depth of sedation to another. There are four defined depths of PSA: minimal sedation (or anxiolysis), moderate sedation, deep sedation, and general anesthesia (Table 140.2). It is important to note that ketamine, a dissociative agent, does not fit neatly on this continuum.
In the ED, the depth of PSA desired depends on the child and the situation. For example, a clinician may choose to administer mild sedation to an anxious child with a nail bed injury in addition to digit nerve block. For a child with a forearm requiring closed reduction, a clinician will likely aim for moderate to deep sedation. Progression from mild sedation or analgesia to general anesthesia cannot be simply divided into discrete stages. In general, as the dose of analgesic and sedative agents increases, consciousness decreases and in most cases the risk of cardiorespiratory depression increases. A child may continue to advance along the sedation continuum until protective airway reflexes are lost and he or she is effectively under general anesthesia. Again, ketamine is unique because airway reflexes and ventilator drive may be preserved despite attainment of the dissociated (sedated) state. It is not always possible to predict how a child will respond to medications. Although the ED physician may intend to achieve mild sedation, moderate or deep sedation may result. Thus, individuals administering moderate or deep sedation and anesthesia should be qualified (and have appropriate credentials) to manage patients at whatever level of sedation or anesthesia is achieved, either intentionally or unintentionally.
Decision to Perform PSA
One of the most important decisions a clinician will make regarding PSA is whether a child is an appropriate candidate. Children encountered in the ED will have a variety of conditions potentially requiring sedation and analgesia. PSA carries risks within itself, most frequently respiratory depression and cardiovascular instability, but potentially others depending on the regimen chosen, and the risks of sedation must be carefully weighed against the anticipated benefits. Factors that must be taken into account when deciding to proceed with PSA include a child’s past medical history and comorbid conditions, prior difficulty with PSA medications, type of procedure, anticipated pain or anxiety, need for immobility, and duration and urgency of the procedure. In order to assess a patient’s degree of current illness, the ASA Classification System (Table 140.3) is used. Assignment of an ASA PS level is not associated with outcome, but rather with a patient’s current condition. It is useful to assign an ASA PS level to children as part of the presedation evaluation. In general, children who are rated ASA PS I and II are appropriate for PSA in the ED. ASA PS III and IV should have consultation with an anesthesiologist barring extremely urgent situations.
TABLE 140.3
AMERICAN SOCIETY OF ANESTHESIOLOGISTS CLASSIFICATION FOR PHYSICAL STATUS LEVEL

Evaluation and Preparation of Patient Prior to Sedation
The ASA recommends that clinicians administering sedation/analgesia should be familiar with aspects of the patient’s medical history that may relate to the medication about to be given. This includes abnormalities of any major organ system; previous personal or family history of adverse experience with sedation analgesia; drug allergies; current medications; time of last oral intake of solids and liquids; and use of alcohol, tobacco, or drugs of abuse. Patients should have a focused physical examination before sedation is administered. This should include at a minimum vital signs, auscultation of the heart and lungs, and evaluation of the pharynx and airway. Conditions that may make endotracheal intubation more difficult (such as short neck, limited cervical spine mobility, small mandible, large tongue, or trismus) should be noted.
For moderate and deep sedation, the ASA recommends providing patients and parents with information on the risks, benefits, and alternatives to sedation and analgesia before a procedure.
Preprocedure Fasting
The importance of preprocedure fasting remains controversial, and it is not known whether preprocedure fasting results in decreased incidence of adverse outcomes. That said, the American Academy of Pediatrics (AAP) recommends several dietary precautions before sedation: children should not have milk or solids for several hours before elective sedation (not within 4 hours for human milk, 6 hours for nonhuman milk, infant formula, or a light meal, and 8 hours for a fatty meal). Intake of clear liquids may continue, but should cease in all cases within 2 hours of the scheduled sedation. Routine oral medications may be taken with a sip of water. ASA noted insufficient evidence to assess the impact of timing of fasting on emesis, reflux or pulmonary aspiration, however, they continue to recommend maintenance of current preprocedure fasting times. ACEP publications have also noted that there is insufficient evidence to fully support these time intervals, and offer a “level B recommendation” (moderate clinical certainty) that “procedural sedation may be safely administered to pediatric patients who have had recent oral intake.”
Clinicians should be aware of other factors besides stomach contents that can increase the risk of pulmonary aspiration. The depth of sedation is an important consideration, as deep sedation may impair (and general anesthesia usually does impair) airway reflexes, making aspiration more likely. Medication choice also plays a role. The dissociative sedation caused by ketamine preserves airway reflexes, and aspiration is much less likely with this agent compared to others. Conversely volatile agents frequently used by anesthesiologists not only impair airway reflexes, but can also cause vomiting, thereby increasing risk of aspiration. Any positive pressure ventilation may increase risk of aspiration in a sedated patient. Patient characteristics that increase the risk for pulmonary aspiration include known difficult airway, extremes of age, higher ASA physical status, and any medical condition that increases risk of gastroesophageal reflux.
Patients with known gastroesophageal reflux or dysmotility disorders may benefit from appropriate pharmacologic treatment to reduce gastric volume and increase gastric pH, although routine use for all patients is not recommended. Similarly, presedation antiemetics have not been shown to reduce pulmonary aspiration and are not recommended for routine use.
For the emergency patient, sedation should still be preceded by an evaluation of food and fluid intake. It is prudent to assume that patients in the ED have full stomachs when planning the use of sedatives or analgesics. When determining preprocedure fasting time, it is important to individualize the decision. Clinicians should consider depth of desired sedation, specific agents used, timing and urgency of procedure, and any conditions that may increase the chance of aspiration. The increased risks of sedation must be weighed against the benefits; the lightest effective sedation should be used. Some patients may benefit from delaying the procedure or administration of appropriate pharmacologic treatment to reduce gastric volume and increase gastric pH. Consider airway protection before emergency sedation.
Personnel
Personnel who are trained in basic life support and the recognition of complications of sedation should be available in the procedure room at all times once sedation has started. One such person should be responsible for monitoring the patient and should not be involved in the procedure itself. Personnel must understand the pharmacology of the sedatives they use. The AAP recommends that an additional person trained in advanced pediatric resuscitation should be available to assist the patient.
Equipment
For children who are undergoing moderate sedation, emergency equipment should be available for children of all ages and sizes. This should include a suction device and a positive pressure oxygen delivery system capable of delivering at least 90% FiO2. In areas where moderate and deep sedation are being administered, advanced airway equipment should be available, including nasopharyngeal airways, oral airways, laryngeal mask airways, and endotracheal tubes and laryngoscope. Reversal agents such as naloxone and flumazenil should be immediately available in the ED. Additional equipment recommendations include a nearby defibrillator and medications that might be needed for resuscitation.
Monitoring
A designated person must continuously observe the child’s face and chest wall motion. Unless truly unavoidable, equipment and special drapes must not block this observation. Patients should have continuous monitoring of oxygen saturation, respiration, and heart rate with (at least) intermittent monitoring of blood pressure during and after the procedure. Continuous electrocardiographic monitoring has not been proven to affect outcome; however, it is simple, readily available, and should be routine. Monitoring with pulse oximetry is essential because of the proven difficulty in recognizing hypoxemia even by experienced personnel.
Measurement of exhaled carbon dioxide (EtCO2) is another modality that has been used extensively in anesthetized patients and has gained a foothold as a sensitive method for monitoring sedated patients in the ED. Noninvasive capnography by nasal cannula is available for pediatric patients; some units combine this nasal cannula device with an oxygen delivery component as well. The visualization of the “sidestream” EtCO2 waveform over time can offer the clinician insight into the ventilatory status of a nonintubated patient who has normal baseline lung function. The specific waveforms elicited by capnography can assist the clinician in determining central apnea, laryngospasm or airway obstruction, bronchospasm, periodic breathing, hyperventilation, and hypoventilation (bradypneic and hypopneic). Monitoring of EtCO2 provides earlier detection of respiratory depression than traditional monitoring techniques. The ASA has long been recommending use of EtCO2 for all general anesthesia cases. Although it is not yet clear which patients require EtCO2 monitoring in the ED, it would be a helpful adjunct for patients receiving deep sedation, for those whose ventilation cannot be observed while receiving moderate sedation, and for those who are receiving supplemental oxygen empirically, thus blunting the already limited ability of the pulse oximeter to detect hypoventilation or apnea.
The bispectral index (BIS) monitor is an electroencephalographic device that is attached to a patient’s forehead and has been introduced as a potential marker of a patient’s sedation level. The device has been widely used for monitoring patients receiving general anesthesia. A patient with a BIS value of 0 has no electrical activity, and a patient with a score of 100 is wide awake. BIS data are useful for trending purposes rather than as an intended target. Unfortunately, specific numerical BIS values may not correlate well with other observational measurements of sedation in children undergoing ED procedures, and can be highly variable at lighter levels of sedation. Additionally, BIS values are unreliable in patients who receive certain sedatives such as ketamine and nitrous oxide. Further research is needed to determine how BIS monitoring can be effectively used in pediatric emergency patients.
If a patient requires deep sedation, it is recommended that an IV line be established before sedation and maintained throughout the procedure and until the patient is no longer at risk for cardiorespiratory depression. If a patient is receiving an intramuscular (IM) agent, the clinician should determine the need for an IV based on the risk for cardiovascular compromise or the potential need for rapid sequence intubation (RSI).
Patients should have continuous monitoring of heart rate, respiratory rate, and pulse oximetry, and, if used, end-tidal CO2 (see above discussion). Monitors can be set to measure blood pressure automatically at specified intervals. At a minimum, vital signs should be documented at baseline, after drug administration, after the procedure is completed, during early recovery, and at the completion of recovery. If deep sedation is anticipated or the child has an underlying illness, the frequency of documentation of vital signs should be increased (e.g., to every 5 minutes). Patients are at highest risk for complications from sedation during the 5 to 10 minutes after administration of the medication and during the period immediately after the procedure when painful stimuli are discontinued. After the procedure, appropriate staff should continue to observe patients who received sedation; continuous monitoring of heart rate and oxygen saturation should continue until the patient has met discharge criteria. Before discharge, any child who has received moderate or deep sedation should be awake enough to speak and sit without assistance and preferably able to ambulate with minimal assistance. Younger children should be able to perform age-appropriate functions. The child should also have adequate hydration status, documentation of stable cardiovascular function, and an adequate airway.
Sedation Protocols
Because of the potential for respiratory depression with the sedative and analgesic agents discussed in this chapter, it is imperative that EDs develop protocols for their use. Several organizations, such as the AAP, the ACEP, and the ASA, have prepared guidelines for sedation in children. These protocols differ in certain fine points, but there is general agreement on major issues. The specifics of the ED protocol should be modified at each individual institution.
Choice of Technique and Medications
Selection of medication is informed by the specific procedure, the patient, and the desired depth of sedation (Table 140.4). For example, the clinician can decide that a child needs some assistance in keeping still, an increased receptiveness to distraction techniques, or amnesia for the procedure. This child requires anxiolysis rather than sedation, and will receive a relatively smaller dose of a medication that will not require cardiorespiratory monitoring. However, a child who needs to be still during a delicate procedure may require a larger dose of the same medication with some risk of cardiorespiratory compromise and, therefore, more intensive monitoring.
For procedures, it is helpful to discuss the sedation plan with the proceduralist. It is important to know the anticipated duration of the procedure, and the time that the child may need to remain immobile. For example, one may decide to use morphine rather than fentanyl if a procedure is expected to last longer than 10 minutes. It is equally important to know the duration and severity of pain usually involved in this procedure. Some procedures, such as fracture reduction, are only brief but very painful and also require the child to remain immobile during the casting portion of the procedure. The same is true for procedures in which the administration of regional anesthesia is painful, but the remainder of the procedure is not. Patients who undergo nonpainful procedures, such as computed tomography (CT) or magnetic resonance imaging (MRI) scans, or those receiving regional analgesia, may be best served by receiving an agent that provides anxiolysis or sedation alone. Finally, one must consider the level of distress that visualization of the procedure may cause for patient and family members. Agents that provide an amnestic effect may be desirable in the case where a child could be frightened by the sight of blood or other body fluid.
TABLE 140.4
INFORMATION THAT CAN ASSIST CLINICIAN IN DETERMINING TYPE OF AGENT NEEDED FOR PROCEDURAL SEDATION AND ANALGESIA
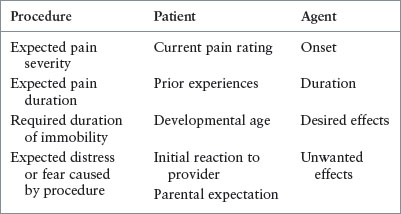
The child’s likely reaction to the procedure can be estimated from his or her current pain rating, developmental level, and initial reaction to healthcare providers. Parents are often the best source of information about the child’s expected reaction to the procedure. Some parents may know that, despite a pain-free procedural technique using topical anesthetic for a facial laceration, their child will be so fearful of strangers that nonpharmacologic techniques will not suffice. Conversely, a mother may express that her child was calm throughout his or her last laceration repair and believe that she can adequately distract him or her during the procedure.
METHODS OF DELIVERY
Delivery route of analgesics and sedatives is an important consideration when choosing an analgesic or PSA regimen. Pharmacologic factors such as drug onset, absorption, and duration of action can affect choice of delivery. The safety of certain medications and regimens should be taken into account; the presence of a working peripheral IV line allows for quick delivery of reversal agents or resuscitation medications. Conversely, while an IV delivery route may be preferred in a certain instance, IV placement may not be possible due to body habitus, vein sclerosis, dehydration, or patient cooperation, among others, and a different delivery route must be considered. The IM route is not ideal because it causes delayed drug absorption and the dosage of drug given cannot be titrated. Subcutaneous (SC) administration can also be an option for certain medications in patients without IV access. Also, pain inflicted on the child must not be ignored; IV, IM, and SC routes are painful. Oral medications can be a good option if there is no contraindication, however the onset of action can be delayed compared with other routes.
The intranasal (IN) delivery route has recently been gaining popularity. IN drugs have several advantages, including faster absorption compared with oral or IM, no first-pass metabolism, and avoidance of a painful needle stick. Some children find the nasal route noxious. IN medications should be administered into the well-vascularized nasal vestibule. Volumes of 0.3 mL or less are easily absorbed, while larger volumes may run off into the posterior pharynx. The ideal medication for the IN route has a low molecular weight, is highly lipophilic, and has neutral pH. Conditions that may affect IN drug absorption include presence of blood or nasal secretions, impaired ciliary function, or anatomical conditions such as polyps. The four main methods to deliver IN medications are dripping, spray, atomization, and nebulization. More research is needed to determine how IN medications can augment care in the pediatric ED.
SEDATIVES
The sedative/hypnotic agents include benzodiazepines, chloral hydrate, pentobarbital, etomidate, propofol, and nitrous oxide (N2O). Barbiturates include pentobarbital, thiopental, and methohexital. Ketamine is considered a dissociative agent and has both sedative and analgesic properties. Dexmedetomidine, an α2-adrenoceptor agonist, also provides both sedation and analgesia. The relative advantages and disadvantages of these agents are listed in Table 140.5.
Benzodiazepines
Some children who are undergoing painless procedures may be too young, anxious, or emotionally labile to remain still enough for the procedure to be successful.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







