A multimodal approach to reduce PONV includes strategies to reduce risk of PONV combined with one or more prophylactic agents. Rescue anti-emetic therapy is indicated in patients complaining of nausea or vomiting in the PACU. First, the patient should be evaluated for treatable causes of nausea or vomiting, such as hypotension or hypoxia.
Then, the rescue drugs are basically the same as the prophylactic drugs, but should be from a different pharmacologic class than the prophylactic drugs previously given to the patient. Also metoclopramide and/or ephedrine may be used for treatment of emesis.
PDNV (post-discharge nausea/vomiting) is particularly important to prevent because these patients are at home and do not have access to intravenous, fast-acting rescue anti-emetics. Among ambulatory surgery patients, the incidence of PONV is 33–50%.[26,30]
The prevention of PDNV involves excellent multimodal analgesia strategies, which would reduce postoperative opioid consumption. Combination therapy with long-acting IV and oral anti-emetic in PACU would also reduce PDNV. In some ambulatory practices, patients at high risk for PDNV are often given a prescription for ondansetron tablets to be taken as long as they are taking opioids for pain control. If identified early in the preoperative interview, patients are given a prescription for a transdermal scopolamine patch to be applied the night before surgery.[31]
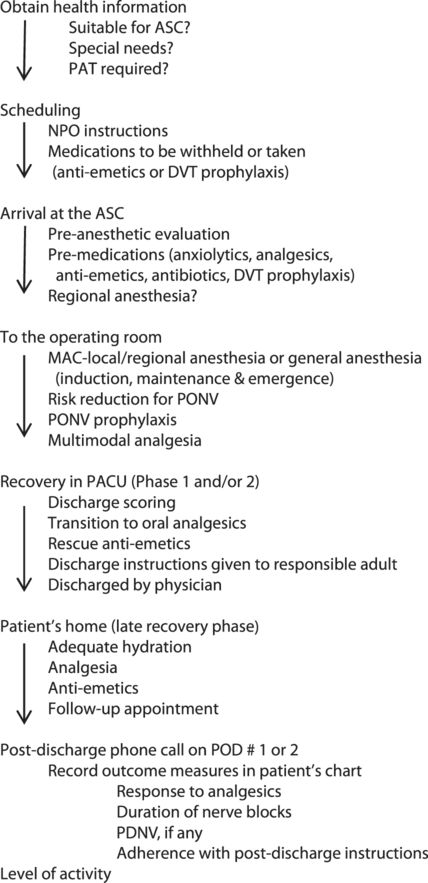
Recovery: the post anesthesia care unit (PACU)
After emergence, tracheal extubation or LMA removal, the patient is transported to the PACU. The goal of a successful ambulatory anesthetic is to have the patient ready for discharge in the shortest possible time. The advantages to this approach are better patient comfort, reduced hospital-acquired infections, and reduced costs.
A patient is ready to be discharged home when certain predetermined discharge criteria are met. These criteria vary among institutions and are patient-dependent. The patient can then continue with Phase III/late recovery in their home environment.
The Aldrete Scoring System[32] is widely used across institutions and is a modification of the APGAR scoring system. It assesses if a patient can transition from Phase I recovery to Phase II recovery. Most institutions use an updated or modified Aldrete Scoring system. Assessment of patient readiness for discharge to home from Phase II recovery is by the Post Anesthesia Discharge Scoring System, PADSS.[33]
| Patient sign | Criterion | Score |
|---|---|---|
| Activity | Able to move 4 extremities* Able to move 2 extremities Able to move 0 extremities | 2 1 0 |
| Respiration | Able to breathe and cough Dyspnea Apneic or obstructed airway | 2 1 0 |
| Circulation | BP ± 20% of pre-anesthesia level BP ± 20–49% of pre-anesthesia level BP ± 50% of pre-anesthesia level | 2 1 0 |
| Consciousness | Fully awake Arousable (by name) Non-responsive | 2 1 0 |
| Oxygen saturation | SPO2 > 92% on room air Requires supplemental O2 to maintain SPO2 > 90% SPO2 < 90% with supplemental O2 | 2 1 0 |
* Or at baseline.
Recovery then continues at home under the care of a responsible adult who has received discharge instructions in the PACU.
Although there are other scoring systems to assess patient recovery, they each have limitations and will not be further elaborated here. Many ambulatory practices use a combination of the Aldrete scoring system and the PADSS.
The patient must score 8 or higher with no zeroes in any category to be discharged to Phase II recovery.
The patient must score 8 or higher with no zeroes in any category to be discharged home.
It is not necessary to require patients to void before discharge. This practice has been shown to cause delayed discharge. If a patient is at high risk for urinary retention, they are observed for an extra hour and residual urine in the bladder is measured via ultrasound (bladder scan). If it is in excess of 400 ml they are catheterized to empty the bladder and then discharged home with clear instruction about when to seek medical assistance (if they do not void further).
| Patient sign | Criterion | Score |
|---|---|---|
| Dressing | Dry and clean Wet and stationary Growing area of wetness | 2 1 0 |
| Pain | Pain-free Mild pain controlled with oral analgesics Severe pain requiring IV analgesics | 2 1 0 |
| Ambulation | Dizzy when upright Dizzy when supine | 2 1 0 |
| Nausea | None Mild nausea Nausea and vomiting | 2 1 0 |
| Vital Signs | BP and pulse ± 20% of pre-anesthesia level BP and pulse ± 20–40% of pre-anesthesia level BP and pulse > 40% of pre-anesthesia level | 2 1 0 |
It is also not necessary to insist that patients drink and retain fluids prior to discharge. The ASA task force on post-anesthetic care practice guidelines recommends that oral fluid intake not be part of a discharge protocol. Forcing oral intake of fluids may actually increase the incidence of PONV.
Fast-tracking is a process whereby patients bypass Phase I recovery and go directly to Phase II from the OR.[34] Not all patients are suitable for fast-tracking.
Scoring systems are tools to assist the practitioner, not a substitute for clinical judgment or common sense. They should therefore be individualized based on comorbidities and social factors.
Discharge from the facility is a physician responsibility. For all patients receiving GA, regional anesthesia, or sedation, an anesthesiologist must be present in the building until the patient is discharged. For patients receiving no sedation and only local anesthesia by the surgeon, it is the surgeon’s responsibility to discharge the patient.
The patient must be discharged into the care of a responsible adult who will remain with the patient overnight. They must be provided with written instructions regarding diet, activity, medications, and follow-up appointments. Circumstances which would require them to seek medical attention should also be clearly outlined, along with the emergency contact information for the surgeon or his/her designee.
Post-discharge follow up
Most ambulatory care practices have a system of follow up where the patient receives a phone call on POD 1 or 2. This is important to ensure patient safety during the late recovery phase. During this call, they are asked about how much pain they experienced, if the pain was relieved or made tolerable by their current dose of oral analgesics, nausea or vomiting episodes if any, and oral intake. They are also asked about how long the regional/local anesthetic lasted, ambulation and the ability to carry out permitted daily activities, fever, and unusual pain or bleeding. Any early postoperative complications are identified and either treated or the patient is directed to seek medical attention. Compliance with postoperative instructions is verified and clarifications are provided.
Give two cooks the same ingredients and the same recipe; it is fascinating to observe how, like handwriting, their results differ. After you cook a dish repeatedly, you begin to understand it. Then you can reinvent it a bit and make it yours. A written recipe can be useful, but sometimes the notes scribbled in the margin are the key to a superlative rendition. Each new version may inspire improvisation based on fresh understanding (David Tanis, Heart of the Artichoke: and Other Kitchen Journeys)
Special considerations
In this section, we will deal with specific groups and their anesthetic management in the ambulatory setting.
Obesity
Obesity is defined as having a Body Mass Index (BMI) of ≥ 30 kg/m2. It is further classified into Class I (BMI 30–34.9 kg/m2), Class II (BMI 35–39.9 kg/m2), and Class III (BMI ≥40 kg/m2). Class III obesity is further subdivided into super-obesity (BMI 50–59 kg/m2) and super-super obesity (BMI ≥ 60 kg/m2).[35] Obesity is a worldwide epidemic and in 2009–2010, the prevalence of obesity was 35.5% among adult men and 35.8% among adult women in the US.[36] Obese patients may present for a wide variety of surgical procedures, which may or may not be linked to their obesity, ranging from outpatient bariatric surgery to cataract surgery.
Preoperative preparation by systems
Respiratory
Check for associated obstructive sleep apnea (OSA). Some patients may already be diagnosed with OSA and using CPAP. Instruct them to bring their CPAP device on the day of surgery and use it in the postoperative period not just at night but also for daytime sleeping.
For patients who are undiagnosed but high risk for OSA (based on the STOP-BANG screening questionnaire),[37] the type of surgery will determine whether they need a sleep study preoperatively. Procedures that are low to intermediate risk, and not likely to be associated with severe postoperative pain, are amenable to loco-regional techniques or non-opioid analgesics for pain management and can be safely performed on obese patients in the ambulatory setting without the need for a sleep study.
Check for the potential for difficult intubation and obtain previous anesthesia records. The neck circumference appears to be the biggest predictor of difficult intubation in morbidly obese patients.[38] These patients are also difficult to mask ventilate. Whether a patient with a known difficult airway should be anesthetized at a free-standing ASC is debatable. This decision should take into account availability of skilled back up to assist the anesthesiologist if necessary and the availability of difficult airway equipment.[39]
These patients tend to have perioperative hypoxemia and require close monitoring. They are also sensitive to the respiratory depressant effects of anxiolytics such as midazolam, which should be administered with caution, in titrated doses.
Cardiovascular
Obese patients tend to have coexisting arterial hypertension and left ventricular diastolic dysfunction. Many of these patients have restricted mobility, making it difficult to assess their functional capacity. They may therefore have undiagnosed coronary artery disease. The need for preoperative cardiac testing depends on the surgical procedure and presence of comorbidities. Most low- to intermediate-risk ambulatory procedures can be carried out without further cardiac testing.
Patients with suspected obesity hypoventilation syndrome are not candidates for outpatient surgery.
Obese patients are at high risk for thromboembolic events and should receive deep venous thrombosis (DVT) prophylaxis perioperatively, and early ambulation must be encouraged after surgery. They may sometimes require DVT prophylaxis for up to 3 weeks postoperatively.[40]
Gastrointestinal
Obesity is frequently associated with gastroesophageal reflux and patients may already be on medications to treat it. These drugs should be continued on the day of surgery. For patients who are not on anti-reflux medications, consider preoperative famotidine or ranitidine to decrease gastric volumes and increase gastric pH. Current fasting guidelines (6 hours for solids, 2 hours for clear liquids) are acceptable in obese patients.[41]
Endocrine
Many of these patients have insulin resistance and diabetes. They should be given appropriate instructions for managing their anti-diabetic regimen on the day of surgery. Blood glucose levels should be checked and managed in the perioperative period.
Miscellaneous
Ensure that these patients have a responsible caregiver to bring them to their surgery, receive discharge instructions, and take them home after surgery. Ensure that the surgery center has the appropriate equipment (stretchers, OR tables) to accommodate the girth and weight of these patients. It can be difficult to obtain IV access in these patients. Ultrasound guidance can be used to locate and cannulate peripheral veins in these situations.
Intraoperative
Loco-regional techniques of anesthesia, accompanied by minimal sedation, are preferred whenever possible in these patients. For patients in whom GA is indicated, the following tips may be useful. Before induction of GA, position these patients head-up or ramped up to raise the head and shoulders. Avoid the supine position. The head-up position improves functional residual capacity (FRC) and allows for a longer period of apnea before desaturation.[39]
Preoxygenate with 100% oxygen with a tight mask with PEEP of 5–10 cm, and have the patient take at least four vital capacity breaths of 100% oxygen before induction of GA. Have difficult airway equipment available in the room. Sedated, topicalized fiber-optic intubation may be indicated in patients who appear difficult to mask ventilate on the preoperative examination. A videolaryngoscope may be used in other patients to facilitate tracheal intubation. Optimal positioning ultimately is the key to successful atraumatic intubation in these patients.[42,43]
Rapid sequence induction may be indicated in patients at risk for aspiration. In these cases, the decision should be made on an individual basis after airway assessment.
Maintenance of anesthesia can be achieved by any of the techniques mentioned earlier in the chapter.
Pay close attention to positioning to avoid nerve injuries in these patients.
Pay attention to the following dose adjustments.
1. Propofol: induction (bolus) dose should be based on ideal body weight and maintenance (infusion) dose should be based on total body weight.
2. Succinylcholine: dose should be based on total body weight due to increased pseudocholinesterase activity with increasing weight and larger extracellular fluid volume.
3. Rocuronium: dose based on ideal body weight. If dosed according to total body weight, expect a longer duration of action.
4. Benzodiazepines: dose according to ideal body weight. They are highly lipophilic and therefore distribute in the fat compartment resulting in prolonged duration of action if dosed according to total body weight.
6. Remifentanil: dose according to ideal body weight. Pharmacokinetics of remifentanil are similar in obese and non-obese patients making this a useful adjunct for TIVA in the obese patient.
7. Dexmedetomidine: dose according to total body weight. In doses of 0.2–0.7 µg/kg/h, it produces effective sedation, analgesia, and minimal respiratory depression making it ideal in these situations.
9. Sugammadex: currently not FDA-approved in the US, but may be useful in obese patients.[44]
The goals of fluid management are maintaining normovolemia and avoiding rapid fluid boluses.
Some tips for effective mechanical ventilation of the lungs in these patients are as follows.
11. No specific ventilatory (volume control or pressure control) mode is better in these patients.
12. Moderate PEEP (5–10 cm H2O) with recruitment measures prevents atelectasis.[47]
13. Higher levels of PEEP could impair venous return and cause hypotension.
14. Titrate FiO2 to maintain acceptable oxygen saturations, avoid 100% oxygen as it could lead to absorption atelectasis.
Emergence
Ensure adequate reversal of neuromuscular blockade. Extubate in the semi-recumbent or semi-sitting or propped-up position to maximize FRC. Administer supplemental oxygen and observe the patient for a few minutes before transporting out of the operating room.
Postoperative
1. Consider using BIPAP or CPAP in the recovery room until the patient is fully awake.
2. Ensure adequate analgesia as stated earlier in the chapter using opioid-sparing techniques.
3. Encourage early ambulation.
4. Patients who are unable to maintain oxygen saturation greater than 95% without supplemental oxygen or non-invasive ventilation should be monitored in the hospital and not discharged home.
5. Patients who are likely to require a significant amount of opioids for pain management are also not candidates for discharge.
6. Both the patient and their caregiver should be given explicit instructions on using CPAP not just at night but anytime the patient is sleeping.
To summarize, perioperative outcomes in obese patients are influenced by the invasiveness of the surgery, the anesthetic technique, the presence of comorbidities, and the BMI (> 50 kg/m2). Ambulatory surgery appears to be safe in patients with BMI < 40 kg/m2 when comorbid conditions are well managed. Patients with BMI between 40 and 50 kg/m2 require thorough preoperative assessment and screening to rule out obesity-related comorbidities (obesity-related hypoventilation, OSA, CAD, pulmonary hypertension, CHF) which would preclude them from ambulatory surgery.[48]
The child (see also Chapter 11, Pediatrics)
Anesthetic care in children usually begins with local anesthesia pads on both hands (i.e., EMLA® cream) to prepare for painless venous access. If the child accepts oral tablets or mixture, an appropriate dose of acetaminophen and/or an NSAID should be administered orally 1 hour or more before surgery for pain prophylaxis. If this is not possible due to the child’s resistance, these drugs may be given IV or rectally after the start of anesthesia. Usually sedative premedication is not needed in a well-assured child accompanied by a close relative. The use of preoperative sedatives may prolong the postoperative recovery and discharge, and also disturb the normal diurnal sleep pattern. Still, in an agitated child, midazolam oral mixture 0.5 mg/kg may be a reasonable option to control the preoperative situation when needed. An alternative may be rectally diazepam suspension, 0.5–1.0 mg/kg.
A quiet and familiar atmosphere around the child can be very helpful for facilitating an uneventful preparation. Clear fluids (except milk products) until 2 hours before the start of anesthesia should be permitted. If surgery is planned after lunch, an early morning light breakfast, up to 6 hours prior to surgery, should be allowed. The child should preferably wait as little as possible in the general waiting area, and preferably with his/her parents surrounded with toys, books, or video screens available in a room furnished with regular rather than hospital-type furniture. It is usually wise to avoid mixing children waiting for surgery with discharge ready children, unless the latter are happy and fully recovered.
For routine inductions, it may be wise to keep the child as normally dressed as possible (including shoes), sitting on a parent’s knee with some toys available and/or a computer screen with cartoons running close by. Although an IV induction may be preferred because it is safer and faster, it may not be possible in smaller children. IV induction can help decrease the chance of aspiration and enable better management of laryngospasm and bradycardia. Even though children undergoing elective ambulatory surgery may be appropriately fasting, they do sometimes have stomach content anyway either because they don’t tell their parents about recent food intake or parents do not tell the provider from fear of surgery cancellation.
If IV placement is planned prior to induction, consider removing the EMLA patch a few minutes prior to IV placement to minimize skin pallor and vasoconstriction, although this is not critical. One can usually show the child, with a gentle squeeze (do it gently, but say that you are squeezing hard!) that the topical anesthesia works. After vein cannulation, a Lactated Ringers or 0.9% normal saline solution should be started, and a pulse oximeter placed. Then, intravenous induction with opioid plus propofol can be performed.
Alternatively, an inhalational induction can be performed, usually with sevoflurane (8% in oxygen until deep sleep), followed by venous access. This technique may be preferred in these cases:
– the child or parent insists (i.e., gentle persuasion does not make them change their minds, for instance due to a good previous experience) on inhalational induction;
– cannulation in the EMLA treated area is unsuccessful, and there are no good veins outside the EMLA-applied area;
– unsuccessful cannulation after two attempts inside the EMLA-applied area or one attempt outside the EMLA-applied area.
In children who did not have EMLA or if the EMLA-applied area does not have a good vein for cannulation, it is reasonable to make one quick attempt. One can tell the child that just before skin penetration they will feel pain briefly, but then they will feel no more pain.
For very short procedures (5–10 min), one can just ventilate the child manually through the anesthesia mask; longer cases may warrant an LMA, if appropriate. For some procedures such as tonsillectomies, adenoidectomies and dental surgery the child will likely need to be intubated. This is because the surgeon will be working in the mouth and may dislodge the LMA, and also, as in the case of tonsillectomy, an endotracheal tube will allow for better control of the airway in case of profuse bleeding. However, in cases where there are experienced surgeon-anesthesiologist teams and in the presence of good communication, an LMA can be used for adeno-tonsillectomies.
There are special considerations in children when propofol is used. Children will require about 50–100% more than adults during induction, and 25–50% more during maintenance, mostly due to pharmacokinetic differences, such as small initial distribution volume and higher clearance (see chapter 4, Pharmacology). For opioids and muscle relaxants the dose will be about the same (per kg) as in adults, although slightly more remifentanil may be needed due to higher drug clearance.
For volatile anesthetics, the MAC values are generally 25–50% higher in children compared to adults, and vary according to the child’s age. If nitrous oxide is available, it may be a useful adjunct in 50% (small children) to 66% concentration in oxygen, in addition to either intravenous or inhalational anesthesia. Nitrous oxide is not a cardiorespiratory depressant in healthy outpatients and has a very rapid on–off effect, and can help reduce the dose of other agents. This can help speed up both induction and emergence from anesthesia. When using nitrous oxide, care should be taken by the end of the case to ensure adequate ventilation with oxygen-enriched breathing gas after turning off the nitrous oxide. Because much of the nitrous oxide is released into the lungs during the first 3–5 min after cessation of administration, a combination of hypoventilation and administration of just air may result in hypoxia during emergence.
Sevoflurane is generally the agent of choice for induction of inhalational anesthesia, and may be used for maintenance of anesthesia. However, sevoflurane is associated with a high incidence (up to 10–20%) of severe, short-lasting agitation during emergence.[49] The incidence will be lower when the child emerges with parents standing close by and having received adequate analgesia. The incidence of emergence agitation may be lower with desflurane, and even lower with propofol.[50] Whereas desflurane is unsuited for inhalational induction, it may be an alternative for maintenance after sevoflurane induction, both due to less postoperative agitation and slightly faster emergence and less respiratory depression.[51] An alternative technique is to turn off the inhalational agent earlier and administer a small bolus dose or short-lasting infusion of propofol towards the end of the case to avoid emergence agitation. The use of propofol can also add a short-lasting anti-emetic effect from propofol.
As in all patients, the surgeon should be encouraged to use local anesthesia as much as possible to decrease postoperative pain, and the anesthesiologist may also consider administering a sacral block with bupivacaine for patients having certain procedures (i.e., hernia, perineal surgery, surgery of external genitalia or lower extremities). Furthermore, non-opioids including NSAIDs may be used, and dexamethasone can help not only with emesis prophylaxis but also with pain reduction. The new COX-2 inhibitors have not yet been approved for use in children, but their use will increase as an alternative to traditional NSAIDs when the latter are contraindicated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree