KEY POINTS
1. Transport from the operating room (OR) to the intensive care unit (ICU) is a critical period for patient monitoring or vigilance. Emergency drugs and airway equipment should be present, and adequate transportation personnel (typically three people) should accompany the patient during transport.
2. Patient “hand-off” to the ICU should be consistent, careful, and structured, and should not distract caregivers from continuous assessment of hemodynamics, oxygenation, and ventilation.
3. Early postoperative respiratory support ranges from full mechanical ventilation to immediate extubation in the OR, depending upon institutional practice patterns, anesthetic techniques, and patient stability. There is no “best” ventilation mode for cardiac surgery patients.
4. Weaning from mechanical ventilation involves assessment of oxygenation adequacy (typically PaO2/FIO2 >100 on positive end-expiratory pressure [PEEP] 5 cm H2O or less), hemodynamic stability, patient responsiveness to commands, and measured ventilatory parameters such as vital capacity and the rapid shallow breathing index (RSBI).
5. Fast-tracking protocols designed to extubate cardiac surgery patients within several hours of completion of surgery are common. With such protocols, early postoperative continuous infusions of propofol or dexmedetomidine may be helpful.
6. Early postoperative differential diagnosis of hypotension is often challenging, and includes hypovolemia, heart valve dysfunction, left ventricular (LV) and/or right ventricular (RV) dysfunction, cardiac tamponade, cardiac dysrhythmia, and vasodilation. Once a diagnosis has been made, optimal therapy usually becomes clear.
7. Hypertension is not uncommon and must be acutely and effectively managed to minimize bleeding and other complications such as LV failure and aortic dissection. The differential diagnosis includes pain, hypothermia, hypercarbia, hypoxemia, intravascular volume excess, anxiety, and pre-existing essential hypertension, among others.
8. Acute poststernotomy pain most often is managed by administering intravenous opioids, but other potentially helpful modalities include nonsteroidal anti-inflammatory drugs, intrathecal opioids, and central neuraxial or peripheral nerve blocks.
9. Early postoperative acid–base, electrolyte, and glucose disturbances are common. They should be diagnosed and treated promptly.
10. Postoperative bleeding may be surgical, coagulopathic, or both. Aggressive diagnosis and treatment of coagulation disturbances facilitates early diagnosis and treatment of surgical bleeding (i.e., return to OR for re-exploration) and avoidance of cardiac tamponade.
11. Discharge from the ICU typically occurs in 1 to 2 days. Criteria vary with cardiac surgical procedures and with institutional capabilities for post-ICU patient care (e.g., stepdown ICU beds vs. traditional floor nursing care).
12. Adequate communication with patients’ family members and adequate family visitation and support greatly facilitate postoperative recovery.

THE PURPOSE OF THIS CHAPTER is to briefly discuss the transport of the cardiac surgery patient from the OR to the ICU, the hand-off of care from the OR team to the ICU team, and an approach to common problems that occur in the first 24 hrs in the ICU. The reader is referred to standard critical care text books for discussion of more chronic ICU problems such as nutrition, infectious disease, sepsis, and multiple organ failure.
I. Transition from operating room to intensive care unit
A. General principles
1. Movement of a critically ill patient in the immediate postoperative period to the ICU or to an intermediate level post-cardiac surgical recovery area is a risky business. Inter- or intrahospital transport of critically ill patients is associated with increased morbidity and mortality [1].
1
2. The American College of Critical Care Medicine (ACCM) guidelines state that “during transport, there is no hiatus in the monitoring or maintenance of a patient’s vital signs” [2].
3. The guidelines state there are four major areas to optimize efficiency and safety of patient transport: Communication (or hand-offs), personnel, equipment, and monitoring. Each of these areas will be discussed.
B. The transport process
1. Prior to movement of the patient from OR table to ICU bed
a. Airway/Breathing. If patients are suitable candidates for fast-tracking (see subsequent section) and meet standard extubation criteria, they can be extubated in the OR, or within 6 to 8 hrs of arrival in the ICU. If the patient is to remain intubated, the endotracheal tube should be checked for position and patency, and should be securely attached to the patient. In addition, all chest tubes and drains should be checked for ongoing bleeding to ensure that immediate transport from the OR is appropriate, and for proper functioning to avoid hemothorax or pneumothorax during transport.
b. Circulation. The patient should be hemodynamically “stable” prior to transport. In general, if the patient requires frequent bolus doses, or increasing doses of vasoactive drugs, it is better to stabilize prior to transport.
(1) Pacemaker. Proper settings and functioning of the pacemaker should be checked at this point (see Chapter 17).
c. Coagulation. Bleeding should be controlled, and a plan for correction of ongoing coagulopathy should be made prior to transport.
d. Metabolic. Metabolic abnormalities (glucose, electrolyte, and acid–base) should be identified and corrected as much as possible prior to the transport.
e. Brief Telephone Report. A brief verbal report to the ICU team should be provided prior to transport (see hand-off section).
f. Special Bed. Patients at high risk for development of pressure ulcers (pre-existing pressure ulcers, poor nutritional status, elderly, poor ventricular function, etc.) should be placed on special beds/mattresses in the OR.
2. Patient movement from the OR table to the transport bed. Movement can cause hemodynamic instability, fluid shifts, and arrhythmias. Movement can also cause inadvertent loss of airway, vascular access, and interruption of intravenous infusions. Residual intracardiac air is a complication of many procedures (e.g., valve replacement) and this air may be easily dislodged when moving the patient. In addition, the position of a pulmonary artery catheter (PAC) can be altered during patient movement. Confirmation of the PAC position (i.e., pulmonary artery waveform rather than pulmonary artery occluded or RV waveform) before and after patient movement should be done. Sudden onset of dysrhythmia should trigger examination of the PAC. Ready access to a large-bore intravenous infusion port and to any ongoing or continuous infusions of medications is critical to managing this period safely and being able to respond promptly.
3. Transport from the OR to the ICU
a. Personnel. Generally, at least three members of the operative team should transport the patient from the OR to the ICU. This should include a member of the anesthesia care team, surgical team, and a nurse or technician. Additional team members (perfusionists, respiratory therapists, etc.) may be needed for patients on mechanical assist devices, inhaled pulmonary vasodilators, or those with acute lung injury (ALI) who require a transport ventilator.
b. Equipment. ACCM guidelines recommend a minimum of a blood pressure monitor, pulse oximeter, and cardiac monitor/defibrillator for all transports of critically ill patients [1]. An additional monitor to consider is continuous end-tidal CO2 for intubated patients. Equipment and drugs for emergency airway management should be immediately available. An oxygen (O2) source with enough O2 for the duration of transport plus 30 min must be available. Basic emergency advanced cardiac life support (ACLS) drugs should be immediately available. All infusions should be checked and all pumps should be fully charged prior to transport. Supplemental O2 should be provided to all extubated patients. Bag mask ventilation (with or without PEEP valves) can be used for most patients. Transport ventilators may be needed for patients with ALI or acute respiratory distress syndrome (ARDS). Mechanical support device batteries should be immediately available.
c. Monitoring. ACCM guidelines state that critically ill patients should “… receive the same level of basic physiologic monitoring and transport as they had in the ICU ….” The same concept applies to patients leaving the OR [1].
d. IV Access. Every effort must be made to avoid a “tangle” of IV tubing. In general, it is best to have one large bore IV identified for rapid administration of fluids or emergency medications. This line should be easily identified and immediately accessible. Bolus medications should ideally be given via a central venous site for faster onset. Finally, all IV fluid bags should be full enough to give fluid boluses as needed.
e. Sedation/Analgesia. In extubated patients, it is best not to give boluses of narcotics during transport. It is probably better and safer to give analgesics prior to transport, then give additional medications after arrival in the ICU. In intubated patients, it is best to start the postoperative sedation and analgesia plan prior to transport to minimize the need to give bolus medications during transport.
II. Transfer of care to the ICU team
A. Importance of hand-offs. The hand-off of care from the OR team to the ICU team is a surprisingly hazardous and dangerous event. The Joint Commission identified that communication failure was the root cause of 65% of sentinel events in 2006 [2]. Numerous studies have shown that the best hand-offs occur when they are structured, standardized, and use checklists [3–6]. Recently, many centers are developing hand-off tools from the electronic medical record [7].
2
B. Logistics. Ideally, each member of the OR and ICU teams should have specific tasks and the hand-off should occur in a standardized sequence [3,4]. One simple sequence would be transition from transport to ICU monitor, then initial ventilator settings, then formal structured hand-off.
C. Transition to ICU monitors. The patient must be continuously monitored during this process. Ideally, each parameter (ECG, O2 saturation, etc.) should be transferred from the transport monitor to the ICU monitor in series, as opposed to unhooking all of them at once then hooking them up one at a time. Some systems allow for all the monitors to be almost instantly switched over by removing the entire “brick” at once. In any event, based on local monitors, there should be an orderly transition between both sets of monitors.
3
D. Initial ventilator settings. Intubated patients must have their endotracheal tube evaluated for patency, security, and position. This can be accomplished with a chest x-ray or with a bedside bronchoscopy. Ventilator parameters including ventilator mode, rate, fraction of inspired oxygen (FiO2), PEEP, and pressure support must be selected. The patients who have no respiratory effort can be placed on assist-control (AC) or synchronized intermittent mandatory ventilation (SIMV) with an adequate rate, tidal volume, and PEEP. The patients who have regained spontaneous respiratory effort can be placed on SIMV or pressure support ventilation (PSV). PSV and SIMV modes can be combined. Excessive use of PEEP impedes venous return and may impair RV performance. The application of PEEP may decrease mediastinal bleeding, although the literature on this topic is inconsistent and this technique must be used with caution, as PEEP’s adverse effects on hemodynamics are well established.
E. The actual hand-off. Once the monitors have been transferred to the ICU bedside monitor and oxygenation and ventilation have been confirmed, a structured hand-off should occur. This should include the patient’s name, age, allergies, medical history, all significant intraoperative events, and the immediate postoperation plan. One structured hand-off form generated from the electronic medical record (EMR) is shown (see Fig. 10.1). Time should be allowed for questions and answers from all members of OR and ICU teams.
Figure 10.1 Structured hand-off form generated from electronic medical records.

1. The initial review of the patient upon his or her arrival to the recovery area includes the patient’s history, age, height, weight, pre-existing medical conditions, any allergies, a list of preoperative medications, and review of the most current laboratory findings (with special emphasis on potassium and hematocrit). The report should include a detailed review of the patient’s cardiac status, including ventricular dysfunction, valvular disease, coronary anatomy, and details of the surgical procedure.
2. An anesthetic review should be presented, which includes types and location of intravenous catheters and invasive monitors, along with any complications that occurred during their placement. A brief description of the anesthetic technique should be discussed to help plan for a smooth emergence. Any difficulties with airway management should be emphasized, particularly when weaning and extubation protocols are utilized. The presence or absence of obstructive sleep apnea should be discussed and the need for continuing patients’ home continuous positive airway pressure (CPAP) or bi-level positive airway pressure (BiPAP) should be addressed. A post-cardiopulmonary bypass synopsis should be reported, including the use of vasoactive, inotropic, and antiarrhythmic drugs, as well as any untoward events such as arrhythmias and presumed drug reactions. This should also include an update on the presence or absence of bleeding prior to chest closure.
3. Early upon arrival to the ICU, the patient’s heart rate, rhythm, and blood pressure should be determined. If the heart is being paced, the settings should be reviewed and all electrodes identified and secured, as the patient may be dependent on the device.
a. If the patient has a permanent pacemaker or defibrillator, the settings should also be reviewed. The devices should be interrogated in the ICU and antitachycardia treatment should be activated. While waiting for the device to be activated, external defibrillator pads should be placed on the patient and a defibrillator should be immediately available [8].
b. If the patient has a ventricular assist device (VAD), the monitor should be attached to a wall-based energy supply and the output of the device should be attached to the display module. The settings of the device and the position and location of the cannula should be reviewed.
c. For patients on extracorporal membrane oxygenation (ECMO), the O2 and air supplies should be attached to the wall outlet supplies, and back-up tanks should be available.
F. Laboratory Tests/electrocardiogram (ECG)/chest radiograph (CXR). After the hand-off is complete and questions are answered, baseline ECG, CXR, and labs should be obtained. An initial arterial blood gas (ABG) should be drawn to ensure the adequacy of oxygenation and ventilation, whether the patient is on a mechanical ventilator or breathing spontaneously. Potassium, blood glucose, and hematocrit levels should be obtained. Acid–base status should be reviewed from ABGs. Baseline coagulation parameters, including prothrombin time (PT), activated partial thromboplastin time (aPTT), and platelet count should be acquired if the patient is bleeding excessively.
III. Mechanical ventilation after cardiac surgery
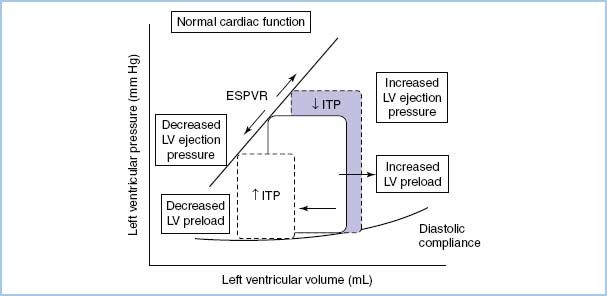
A. Hemodynamic response to positive-pressure ventilation (PPV). Heart–lung interactions of PPV are complex [9,10]. In patients with normal LV function, PPV increases intrathoracic pressure (ITP), which reduces venous return, afterload, and stroke volume (SV) and cardiac output (CO) (see Figure 10.2). On the other hand, in patients with LV dysfunction, decreased preload and afterload actually can improve LV performance and CO (see Fig. 10.3). PEEP further increases ITP and decreases venous return.
Figure 10.3 The effect of increasing and decreasing intrathoracic pressure (ITP) on the LV pressure–volume loop of the cardiac cycle in congestive heart failure when LV contractility is reduced and intravascular volume is expanded. The slope of the LV ESPVR is proportional to contractility. The slope of the diastolic LV pressure–volume relationship defines diastolic compliance [10].
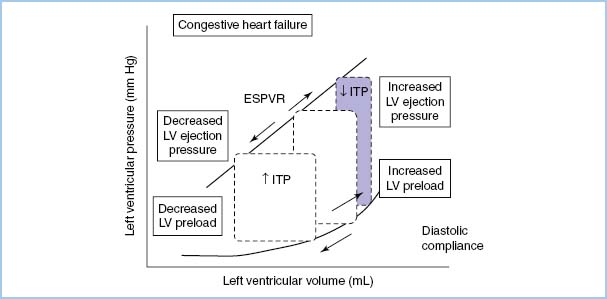
Figure 10.2 The effect of increasing and decreasing intrathoracic pressure (ITP) on the pressure-volume loop of the cardiac cycle. The slope of the LV end-systolic pressure–volume relationship (ESPVR) is proportional to contractility. The slope of the diastolic LV pressure–volume relationship defines diastolic compliance [10].
B. Pulmonary changes after sternotomy and thoracotomy. Cardiac surgery requires either a midline sternotomy or a thoracotomy. Both of these approaches temporarily compromise the function of the thoracic cage, which acts as a respiratory pump. One week after cardiac surgery, there is a significant reduction in total lung capacity, inspiratory vital capacity, forced expiratory volumes, and functional residual capacity compared to preoperative values [11]. Even at 6 wks postoperatively, total lung capacity, inspiratory vital capacity, and forced expiratory volume remained significantly below preoperative values. These findings suggest a marked tendency toward postoperative atelectasis and the possibility of hypoxemia from increased physiologic shunting. These changes in chest wall function can increase physiologic shunt to as much as 13% (compared to a baseline normal value of 5%).
In addition to these changes in mechanics and volumes, there are also abnormalities in gas exchange, compliance, and work of breathing [12]. The cause of these abnormalities is multifactorial and may include inflammation, reperfusion, and other mechanisms.
C. Choosing modes of ventilation
1. Extubated patient. If the patient was extubated in the OR, supplemental oxygen may be all that is necessary postoperatively. Following a general anesthetic, patients will exhibit a mild increase in the PaCO2. Aggressive pulmonary toilet and frequent incentive spirometry must be performed to prevent the atelectasis and hypoxemia that may develop from changes in chest wall function.
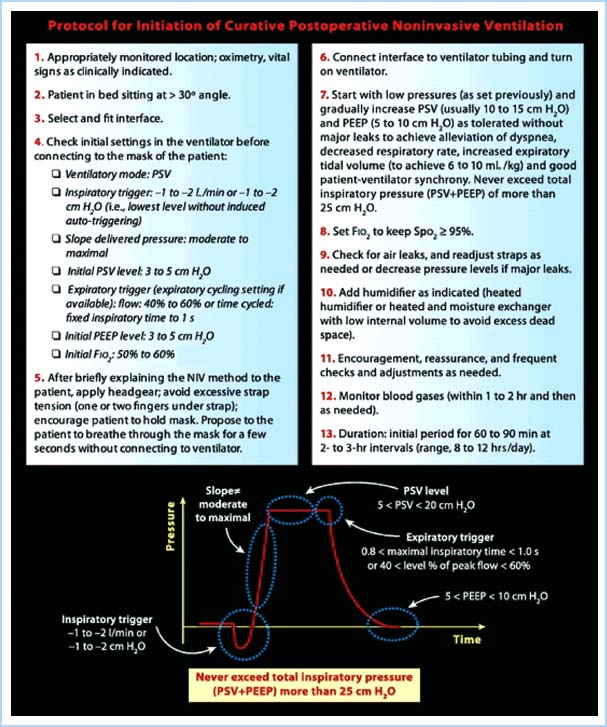
2. Noninvasive ventilation (NIV). NIV can be used to treat or prevent postoperative respiratory failure, and has been shown to prevent reintubation, decrease ventilator-associated pneumonia, and improve outcomes [13,14]. A sample protocol for the use of NIV is shown in Figure 10.4. Contraindications are shown in Table 10.1. Two types of NIV are commonly used:
Table 10.1 Contraindications to noninvasive positive-pressure ventilation
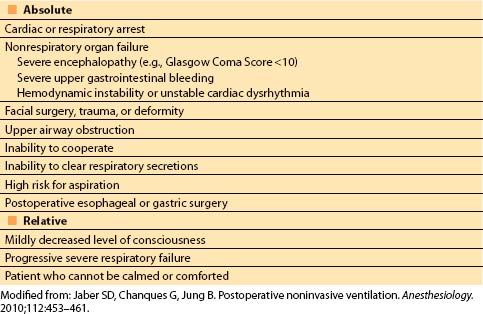
Figure 10.4 Protocol for initiation of curative postoperative NIV. PSV, pressure support ventilation; PEEP, positive end expiratory pressure. FiO2, fraction of inspired oxygen; Spo2, pulse oximetry saturation [13].
a. CPAP, where constant airway pressure is applied during both inspiration and expiration.
b. BiPAP, where PSV is applied during inspiration and PEEP is applied during expiration.
3. Intubated patient
a. If a patient returns from the OR with an endotracheal tube in place, an individualized plan of care should be developed for that patient. The choice of mechanical ventilation mode is based on the patient’s inherent respiratory effort. If a patient demonstrates an inspiratory effort, PSV or SIMV can be used.
If a patient is not demonstrating spontaneous respiratory effort, AC or SIMV should be selected. In AC, a set respiratory rate is delivered regardless of the patient’s respiratory effort. If a spontaneous breath is initiated, the ventilator detects the trigger and delivers a set tidal volume (or pressure if on pressure control ventilation). In SIMV, a set respiratory rate is also delivered, but spontaneous breaths over the set rate are not fully supported (like they are in AC), but are dependent on the patient’s effort.
b. Patients with severe hypoxemia, respiratory failure, ALI, or ARDS will need to be ventilated in a way that minimizes or avoids further “ventilator-induced lung injury” [15]. There have been several recent reviews on the ICU management of ARDS and ALI [16–20].
Initial ventilator settings in patients with ALI or ARDS would include (ardsnet.org):
(1) Any ventilator mode.
(2) Tidal volume (VT) 8 mL/kg predicted body weight.
(3) Set respiratory rate (RR) so minute ventilation is adequate.
(4) Adjust VT and RR to achieve a goal pH 7.30 to 7.40 and plateau pressure <30 cm H2O.
4. Weaning from mechanical ventilation is multifactorial. In many postoperative environments, this can best be accomplished by using an algorithm so that weaning can proceed methodically and without interruption. Figure 10.5 shows an algorithm that could facilitate efficient weaning. Prior to attempts at weaning, the following parameters must be met:
Figure 10.5 Protocolized flow chart for ventilator discontinuation [22]. SBT, spontaneous breathing trial.
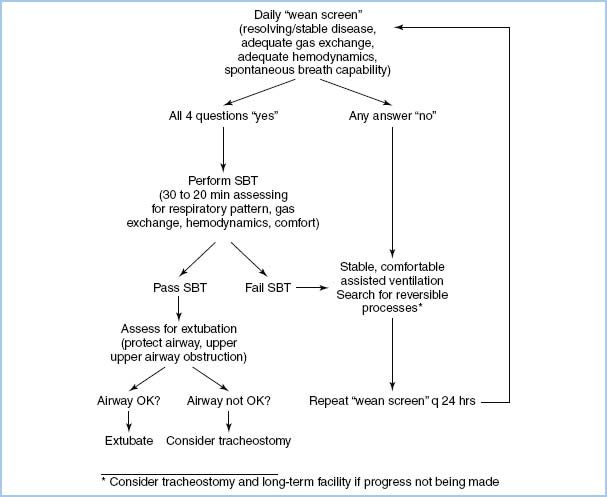
a. Normothermia.
b. Hemodynamically stable.
(1) Stable vasoactive drug requirements.
(2) Not requiring increasing doses or boluses of inotropes or vasopressors.
c. Stable heart rate and rhythm.
d. Normal acid–base and metabolic state.
e. Not bleeding excessively (criteria vary, but generally <150 mL/hr chest tube drainage).
If these criteria are met, the patient is ready to be liberated from mechanical ventilation.
D. Liberation from mechanical ventilation
1. Current recommendations are that patients should be liberated from mechanical ventilation as quickly as possible, and an attempt should be made at least daily [21,22].
2. The first step is to assess the following to determine “readiness to wean”:
4
a. PaO2/FiO2 > 200 mm Hg with PEEP ≤ 5 cm H2O.
b. Hemodynamically “stable.”
c. Awake, alert, and following commands.
d. Able to cough effectively.
e. Adequate reversal of neuromuscular blockade (negative inspiratory force [NIF] of 30 cm H2O or more, able to lift head off bed >5 s, no fade on train of 4, vital capacity >15 mL/kg, etc.).
f. An RSBI (RR/VT in L) < 80 to 100 breaths/min/L after a 2- to 3-min spontaneous breathing trial.
3. If the patient passes the readiness to wean screen, a trial of spontaneous ventilation via T-piece or with low levels of PSV (5 to 7 cm H2O) and PEEP (≤7 cm H2O) for 30 to 120 min is done. At the end of the of the trial, if the RSBI is <80 to 100, the patient should be considered for extubation, if they meet the following final criteria:
a. Awake and alert.
b. Able to cough and clear secretions.
(1) The patients who require suctioning more often than every 2 hrs are at a higher risk for reintubation.
c. No airway edema (as judged crudely by edema of tongue and presence of leak when endotracheal tube cuff is deflated).
d. Hemodynamically stable.
(1) Less than 10% to 20% change in HR, BP, pulmonary artery pressures, cardiac index, etc. during the trial.
e. Normal oxygenation and ventilation.
4. If the patient meets the criteria, extubation can be performed.
5. If the patient does not meet the criteria, correctable causes need to be identified and optimized prior to another attempt.
6. Patients who repeatedly fail spontaneous breathing trials may require more long-term weaning from mechanical ventilation [21].
E. Incentive spirometry, deep breathing, and coughing maneuvers. Patients must be encouraged to use incentive spirometry and to do deep breathing and coughing maneuvers after extubation to reduce atelectasis. There are numerous physiologic causes of hypoxemia. Diffusion abnormality, low FiO2, hypoventilation, and V/Q mismatch along with shunt comprise the list of possibilities, with atelectasis (causing shunt) being the most common. If hypoxemia persists and atelectasis is the presumed cause, NIV can be used to improve oxygenation and decrease shunt.
IV. Principles of fast-tracking
A. Goals of fast-tracking. Fast-track (FT) cardiac surgery was introduced to speed recovery and increase efficiency of limited resources (ICUs). Early extubation, ambulation, cardiac rehabilitation, and discharge are key goals of an FT program. Numerous randomized controlled trials have shown FT cardiac surgery is safe and less expensive than conventional cardiac anesthesia [23]. Initially, FT protocols were limited to young, low-risk patients; however, it can be used safely in older and higher risk patients as well [24].
5
B. Methods of fast-tracking. A variety of anesthetic techniques can be used to facilitate fast-tracking. Shorter-acting intravenous narcotics can be combined with intrathecal opioids to enhance postoperative analgesia [25,26]. Propofol infusions are often used because of a predictable and rapid recovery profile that is almost independent of the duration of infusion. This property makes propofol a very good sedative agent in the early postoperative management of FT cardiac surgery patients, assuming that hemodynamic stability is not compromised by its use. Caution is needed when short-acting agents are used to set the stage for early extubation, as the incidence of intraoperative awareness may be as high as 0.3% [27]. Dexmedetomidine is an intravenous a-2 adrenergic agonist that may facilitate fast-tracking in cardiac surgical patients. Dexmedetomidine possesses both sedative and analgesic properties, and allows patients to follow commands despite adequate sedation, and it most often does not require weaning prior to extubation. Dexmedetomidine does not possess reliable amnestic properties.
Dexmedetomidine has been evaluated in a number of trials in the ICU in both cardiac surgery and non-cardiac surgery patients. When compared to propofol, midazolam, and morphine in separate trials, dexmedetomidine has been shown to provide adjunctive analgesia, induce less delirium, and decrease the duration of mechanical ventilation [28–31].
C. Fast-tracking in the postanesthesia care unit. Many institutions prepare for the postoperative management of cardiac surgery patients by developing enhanced step-down or postanesthesia care units (PACUs), where postoperative management can occur safely and efficiently. These units require nurses who understand fast-tracking techniques, so that patients who have undergone cardiac surgery can move smoothly through early extubation in preparation for early transfer to a regular nursing unit. These specialized PACUs can be very effective in providing FT techniques because of their focused effort in caring for FT cardiac surgery patients [32]. Some investigators have even implemented ambulatory cardiac surgery programs [33]!
D. Utilizing protocols. Developing and utilizing institution-specific FT protocols revolves around systematic plans for weaning patients from ventilators and managing routine postoperative issues to facilitate the progression toward early ICU and hospital discharge. Protocols ideally address most issues before they occur. Fast-tracking protocol development should involve all members of the perioperative care team before implementation.
V. Hemodynamic management in the postoperative period
A. Monitoring for ischemia. Ischemia can be detected by utilizing a continuous ECG with ST segment analysis, although there is a slight delay in diagnosis of ischemia using this method. Many bedside ECG monitoring systems have ST-segment analysis built into their software algorithms, which is a cost-effective method of monitoring for ischemic events. It is important to ensure that the ECG is in diagnostic mode when evaluating potentially ischemic ECG changes. In monitor mode, the ECG filters out some electrical input (to decrease artifact) and may not accurately reflect ischemic changes. If continuous ST segment analysis is chosen, Leads II and V4 or V5 should be monitored, and sensitivity improves if three leads are used (Leads I, II, and V4 or V5, or Leads II, V4, and V5). Other indicators of myocardial ischemia include pulmonary artery pressures and CO, which tend to be less reliable and oftentimes late markers of myocardial ischemia. Transesophageal echocardiogram (TEE) segmental wall-motion abnormalities represent the most sensitive early detector of myocardial ischemia, but continuous monitoring usually is not done because the TEE probe (if used) typically is removed at the end of surgery. It is extremely important for the intensivist to recognize the changes in ECG and hemodynamics that can result from temporary epicardial ventricular pacing. Epicardial ventricular pacing can mimic septal wall dyskinesis that actually represents a pacemaker-induced change in the ventricular depolarization sequence.
Intraoperative and ongoing postoperative ischemia can be detected as soon as 6 hrs after the event begins by examining some specific cardiac markers. The earliest and most useful marker is cardiac troponin I (cTnI). The ability to measure cTnI is particularly useful in cases where ECG monitoring is difficult to interpret, such as with left bundle branch block or LV hypertrophy. Elevated plasma levels of this biologic marker provide clear evidence of ischemia and may suggest a diagnosis of myocardial infarction.
In postoperative cardiac surgical patients, all of the above methods have significant problems. Most often, myocardial ischemia is suspected by ECG changes or unexpected increases in vasoactive drug requirements. The diagnosis is best confirmed by TTE or TEE. Diagnosis may require cardiac catheterization. Treatment options include returning to the OR or medical management.
B. Ventricular dysfunction after cardiac surgery. In addition to pre-existing ventricular dysfunction, postoperative causes of ventricular dysfunction include inadequate myocardial protection, myocardial stunning, incomplete revascularization, and reperfusion injury. Preoperative predictors of postoperative ventricular dysfunction include cardiac enlargement, advanced age, diabetes mellitus, female gender, high LV end-diastolic pressures at cardiac catheterization, small coronary arteries (for coronary revascularization procedures), and ejection fraction less than 0.40. Intraoperative predictors include longer cardiopulmonary bypass (CPB) and aortic cross-clamp times. These factors increase the likelihood of needing inotropic support in the postoperative period. The patients who have normal preoperative cardiac performance and short periods of CPB have a much lower likelihood of requiring postoperative inotropic support. The patients who fail to achieve adequate hemodynamics even with pharmacologic support will require mechanical cardiac assistance such as an intra-aortic balloon pump (IABP) or VAD. Recently, Hollenberg has written an excellent review article on vasoactive drugs in circulatory shock [34].
The myocardium has both b1-adrenergric receptors and b2-adrenergric receptors, which contribute to inotropy and lusitropy (enhanced diastolic relaxation). The b-adrenergic agonists (b-agonists) are often the first-line agents used when there is a need to improve ventricular function after CPB. Depletion of endogenous catecholamines and the resulting b-receptor downregulation can blunt the response to b-agonists. Increased G-inhibitory proteins, reperfusion injury, tachycardia, incomplete revascularization, nonviable myocardium, preoperative use of b-agonists, and acute or chronic heart failure also may attenuate the response to b-agonists.
The inotropic response to b1/b2-adrenergic receptor stimulation occurs via activation of the Gs protein and adenylyl cyclase leading to increased intracellular cyclic adenosine monophosphate (cAMP). It is important to recognize that lusitropy is an active, energy-consuming process; impaired ventricular relaxation (diastolic dysfunction) can cause heart failure alone or in combination with systolic dysfunction. Until recently, there have been no head-to-head clinical trials comparing the clinical outcomes of inotropes and vasopressors. In a trial of 30 patients with dopamine-resistant cardiogenic shock, the patients were randomized to receive either epinephrine alone or dobutamine in combination with norepinephrine. Both groups experienced an increase in cardiac index and a decrease in their creatinine. The group receiving epinephrine experienced more arrhythmias, transient lactic acidosis, and a decrease in splanchnic perfusion [35].
Phosphodiesterase Type III inhibitors (amrinone and milrinone) augment b-adrenergic–mediated stimulation by inhibiting the breakdown of cAMP. Phosphodiesterase inhibitors (PDEIs) act either additively or synergistically with b-adrenergic agonists. PDEIs appear to have anti-ischemic effects and may favorably alter myocardial oxygen consumption [36]. PDEIs can be added to b-agonist therapy or employed as a first-line inotrope. Because PDEIs also induce systemic and pulmonary vasodilation (sometimes termed inodilators), clinical paradigms that favor their use include pulmonary hypertension, RV failure, aortic or mitral valvular regurgitation, and acute/chronic b1/b2-adrenergric receptor desensitization (long-standing CHF, use of b-agonist therapy preoperatively).
Levosimendan is a novel inotrope that, at this time, is neither FDA-approved nor available in North America. Levosimendan is a myofilament “calcium-sensitizer,” which results in increased inotropy by improving the efficiency of the coupling of force-generating myocyte proteins in response to a given level of calcium [37]. Like PDEIs, levosimendan may augment inotropy without significantly increasing myocardial oxygen consumption, thus improving the myocardial oxygen supply/demand balance. In a recent meta-analysis analyzing the use of levosimendan in postoperative coronary artery bypass grafting (CABG), levosimendan was associated with improved mortality and morbidity [38].
B-type natriuretic peptide (nesiritide) has been favored by some for the medical management of CHF [39–42], although some work also associates the use of this agent with increased mortality in that setting. The role of nesiritide in the cardiac surgical population remains ill-defined.
In patients with severely impaired cardiac performance, additional monitoring may be required to ascertain if the patient has optimal myocardial function. Oximetric PACs can provide real-time determinations of mixed venous oxygen saturation (SVO2). A normal SVO2 value of 75% corresponds to a PaO2 of approximately 40 mm Hg. Reductions in SVO2 result from either decreased oxygen delivery (decreased CO, decreased hemoglobin concentration, or decreased arterial oxygen saturation) or increased oxygen consumption. A sustained SVO2 below 40% is associated with increased morbidity and mortality. Similarly, some practitioners choose to use PACs with continuous cardiac output (CCO) determination in such patients, or with both SVO2 and CCO.
C. Fluid management. Managing postoperative fluids after cardiac surgery can be challenging [43]. The effects of hypothermia (vasoconstriction) and hyperthermia (vasodilation) commonly complicate fluid management especially in the first few hours after cardiac surgery. Central venous pressure (CVP) and PAC are frequently utilized in the cardiac surgical population; however, it is imperative to recognize that these monitors measure pressure as a surrogate estimate of volume and/or cardiac performance. The use of filling pressures (CVP, PAOP, PADP) are poor predictors of assessing total blood volume and volume responsiveness [44]. Volume responsiveness is defined by an increase in cardiac index of >15% in response to a fluid challenge. For mechanically ventilated patients who have a regular respiratory pattern, the pulse pressure variation on an arterial pressure waveform can be a highly useful tool in predicting a hypotensive patient’s response to a fluid challenge [45,46].
Cardiac surgical procedures, especially those involving CPB, typically result in fluid sequestration into the interstitial compartment. In addition to the changes in circulating blood volume from blood loss and other factors, fluid shifts into or out of the interstitial or the intracellular compartments can be anticipated in the hours following cardiac surgery. Most cardiac surgery patients reach the recovery area with excess total body fluids present that must eventually be mobilized. Healthy patients who have adequate cardiac and renal function typically diurese these fluids over the first two postoperative days without assistance. Other cardiac surgery patients, such as the elderly or those with renal or cardiac dysfunction, may require diuretic drugs (or possibly dialysis or hemofiltration) to remove excess body water.
Management of blood components, particularly packed red blood cells (PRBC), in the cardiac surgical population is controversial [47,48]. Both transfusion of blood products and anemia are associated with increased perioperative morbidity and mortality. Even though cardiac surgical patients frequently require allogeneic blood products, establishing a transfusion trigger is difficult. Most patients probably require transfusion of PRBC at a hematocrit less than or equal to 21% (hemoglobin less than or equal to 7 g/dL) and few or none require transfusion when the hematocrit is greater than or equal to 30% (hemoglobin greater than or equal to 10 g/dL).
6
D. Managing hypotension. Systematic evaluation of preload, afterload, contractility, and heart rate and rhythm should be performed in the hypotensive patient. If preload is adequate and an acceptable heart rate and a normal cardiac rhythm are present, hypotension represents either inadequate myocardial function or vasodilation. Inadequate cardiac function is managed with inotropes. Vasodilation is managed with vasoconstrictors.
1. Vasodilatory Shock. Eight percent of cardiac surgery patients experience refractory vasodilatory shock after CPB. This refractory shock is associated with increased mortality (25% mortality in one case series). These patients do not respond to traditional treatments, vasopressors, or volume expansion. The causes of the problem are usually multifactorial: Long bypass run, preoperative use of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers, calcium channel blocker agents, heart transplantation, VAD placement, and myocardial dysfunction. Small clinical trials have shown improved morbidity and mortality in this patient population with the use of an intravenous bolus of methylene blue followed by a continuous infusion [49]. Arginine vasopressin may also be useful in this patient population.
Two other unique causes of hypotension that are difficult to diagnose without the aid of transesophageal echocardiography are systolic anterior motion (SAM) of the mitral valve and cardiac tamponade.
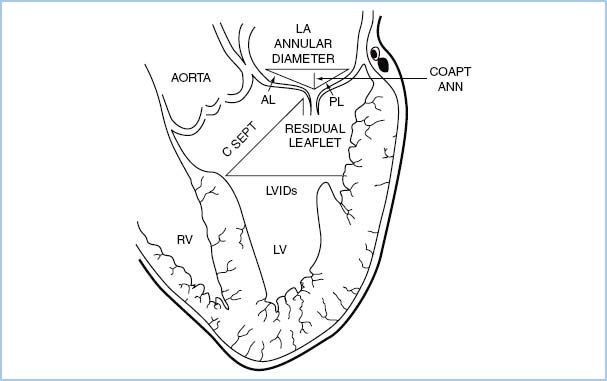
2. SAM of the mitral valve should be assessed in the OR in patients undergoing mitral valve repair or septal myectomy. The c-sept distance (see Fig. 10.6) of 2.5 cm or less is associated with a high risk of SAM [50].
Figure 10.6 Schematic demonstrating the transesophageal echocardiographic measurements performed prior to and after mitral valve repair. The biplane image, obtained from the esophageal location at zero degrees, includes the left atrium (LA), left ventricle (LV), mitral valve, and the LV outflow tract. Lengths of the anterior and posterior leaflets were obtained using the middle scallops. AL, anterior leaflet length; CoaptAnn, distance from the mitral coaptation point to the annular plane; CSept, distance from the mitral coaptation point to the septum; LVIDs, LV internal diameter in systole; PL, posterior leaflet length [50].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








