Chapter 32 Pneumonia
The management of pneumonia is based on four findings and premises:
The net result is that the differential diagnosis is wide and treatment should be started before the aetiological agent is known. The differential diagnosis and the likely causative organisms can be narrowed by using epidemiological clues, the most important of which are whether the pneumonia is community-acquired or health care-associated and whether the patient is immunocompromised. Note that the flora and antibiotic resistance patterns vary from country to country, hospital to hospital and even intensive care unit (ICU) to ICU within a hospital6 and this must be taken into account.
COMMUNITY-ACQUIRED PNEUMONIA
Recent evidence-based guidelines have been issued by the Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS)7 and the European Respiratory Society.8 Links to these and other pneumonia-related guidelines can be found at the following link page: http://www.aic.cuhk.edu.hk/web8/Pneumoniaguidelines.htm.
DEFINITION
Community-acquired pneumonia is an acute infection of the pulmonary parenchyma that is associated with at least some symptoms of acute infection, accompanied by an acute infiltrate on a chest X-ray (CXR) or auscultatory findings consistent with pneumonia (e.g. altered breath sounds, localised crackles) in a patient not hospitalised or residing in a long-term care facility for ≥ 14 days prior to the onset of symptoms.9
AETIOLOGY
Table 32.1 gives possible aetiological agents based on epidemiological clues. Streptococcus pneumoniae is the most commonly isolated organism. The next most common pathogens in patients admitted to ICU are: Legionella species, Haemophilus influenzae, Enterobacteriaceae species, Staphylococcus aureus and Pseudomonas species.10
Table 32.1 Possible aetiological agents based on epidemiological clues2,3,7,9
| Exposure | Organism |
|---|---|
| Exposure to animals | |
| Handling turkeys, chickens, ducks or psittacine birds or their excreta | Chlamydia psittaci |
| Exposure to birds in countries in which avian flu has been identified in birds | Influenza A H5N1 |
| Handling infected parturient cats, cattle, goats or sheep or their hides | Coxiella burnetii |
| Handling infected wool | Bacillus anthracis |
| Handling infected cattle, pigs, goats or sheep or their milk | Brucella spp. |
| Insect bite. Transmission from rodents and wild animals (e.g. rabbits) to laboratory workers, farmers and hunters | Francisella tularensis |
| Insect bites or scratches. Transmission from infected rodents or cats to laboratory workers and hunters | Yersinia pestis |
| Contact with infected horses (very rare) | Pseudomonas mallei |
| Exposure to mice or mice droppings | Hantavirus |
| Geographical factors | |
| Immigration from or residence in countries with high prevalence of tuberculosis | Mycobacterium tuberculosis |
| North America. Contact with infected bats or birds or their excreta. Excavation in endemic areas | Histoplasma capsulatum |
| South-west USA | Coccidiodes species, Hantavirus |
| USA. Inhalation of spores from soil | Blastomyces dermatitidis |
| Asia, Pacific, Caribbean, north Australia. Contact with local animals or contaminated skin abrasions | Burkholderia pseudomallei |
| Host factors | |
| Diabetic ketoacidosis | Streptococcus pneumoniae, Staphylococcus aureus |
| Alcoholism | Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae, oral anaerobes, Mycobacterium tuberculosis, Acinetobacter spp. |
| Chronic obstructive pulmonary disease or smoking | Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, Chlamydia pneumoniae, Legionella spp., Pseudomonas aeruginosa |
| Sickle-cell disease | Streptococcus pneumoniae |
| Pneumonia complicating whooping cough | Bordatella pertussis |
| Pneumonia complicating influenza | Streptococcus pneumoniae, Staphylococcus aureus |
| Pneumonia severe enough to necessitate artificial ventilation | Streptococcus pneumoniae, Legionella spp., Staphylococcus aureus, Haemophilus influenzae, Mycoplasma pneumoniae, enteric Gram-negative bacilli, Chlamydia pneumoniae, Mycobacterium tuberculosis, viral infection, endemic fungi |
| Nursing-home residency | Treat as health care-associated pneumonia |
| Poor dental hygiene | Anaerobes |
| Suspected large-volume aspiration | Oral anaerobes, Gram-negative enteric bacteria |
| Structural disease of lung (e.g. bronchiectasis, cystic fibrosis) | Pseudomonas aeruginosa, Burkholderia cepacia, Staphylococcus aureus |
| Lung abscess | Community-acquired methicillin-resistant Staphylococcus aureus, oral anaerobes, endemic fungi, Mycobacterium tuberculosis, atypical mycobacteria |
| Endobronchial obstruction | Anaerobes, Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus |
| Intravenous drug addict | Staphylococcus aureus, anaerobes, Mycobacterium tuberculosis, Streptococcus pneumoniae |
| Others | |
| Epidemic | Mycoplasma pneumoniae, influenza virus |
| Air-conditioning cooling towers, hot tubs or hotel or cruise ship stay in previous 2 weeks | Legionella pneumophilia |
| Presentation of a cluster of cases over a very short period of time | Bioterrorist agents: Bacillus anthracis, Franciscella tularensis, Yersinia pestis |
INVESTIGATIONS7,8
Investigations should not delay administration of antibiotics as delays are associated with an increase in mortality.3 Important investigations include:
Table 32.2 Organisms which are virtually always pathogens when recovered from respiratory secretions9
| Legionella |
| Chlamydia |
| Tuberculosis |
| Influenza, para-influenza virus, respiratory syncytial virus, adenovirus, Hantavirus, severe acute respiratory syndrome (SARS), coronavirus |
| Stronglyloides stercoralis |
| Toxoplasma gondi |
| Pneumocystis carinii |
| Histoplasma capsulatum |
| Coccidiodes immitis |
| Blastomycoses dermatitidis |
| Cryptococcus neoformans |
Table 32.3 Risk factors for pulmonary tuberculosis
| Living in or originating from a developing country |
| Age (< 5 years, middle-aged and elderly men) |
| Alcoholism and/or drug addiction |
| Human immunodeficiency virus (HIV) infection |
| Diabetes mellitus |
| Lodging-house dwellers |
| Immunosuppression |
| Close contact with smear-positive patients |
| Silicosis |
| Poverty and/or malnutrition |
| Previous gastrectomy |
| Smoking |
Other investigations should be considered in patients with risk factors for infection with unusual organisms. Bronchoalveolar lavage may be useful in immunocompromised patients, those who fail to respond to antibiotics or those in whom sputum samples cannot be obtained.11
MANAGEMENT
ANTIMICROBIAL REGIMES
Each unit should have its own regimens tailored to the local flora and antibiotic resistance patterns. In the absence of such regimens the regimen outlined in Figure 32.1 may be helpful. This should be modified in the light of risk factors (see Table 32.1). Quinolones may be less appropriate in areas with a high prevalence of TB as their use may mask concurrent TB infection. Appropriate antimicrobial therapy should be administered within 1 hour of diagnosis.8,14 There is controversy regarding the appropriate change to empiric therapy based on microbiological findings.7,8 Changing to narrower-spectrum antimicrobial cover may result in inadequate treatment of the 5–38% of patients with polymicrobial infection. Furthermore, dual therapy may be more effective than monotherapy, even when the identified pathogen is sensitive to the agent chosen, particularly in severely ill patients with bacteraemic pneumococcal pneumonia.15 For the treatment of drug-resistant Streptococcus pneumoniae, the regimes in Figure 32.1 are probably suitable for isolates with a penicillin minimum inhibitory concentration (MIC) < 4 mg/l.7 If the MIC is ≥ 4 mg/l an antipneumococcal fluoroquinolone, vancomycin, teicoplanin or linezolid should be given.8
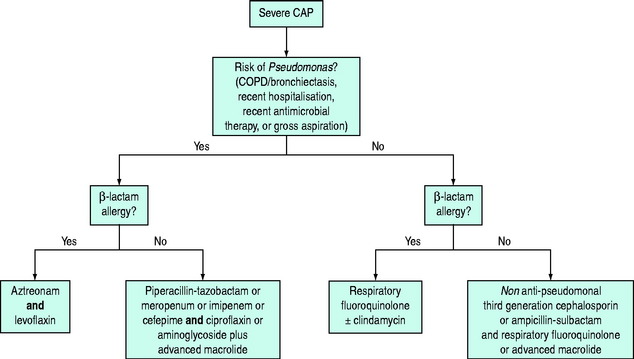
Figure 32.1 Antibiotic regimes for treatment of severe community-acquired pneumonia in critically ill patients.7,8 Respiratory fluoroquinolones include moxifloxacin and levofloxacin. Advanced macrolides include azithromycin and clarithromycin. Cefotaxime is a suitable non-antipseudomonal third-generation cephalosporin.
The role of zanamivir and oseltamivir in severe influenza pneumonia is not clear but early treatment of patients with less severe symptoms results in a reduction of the duration of symptoms if treatment is started early (< 48 hours from onset).16 Oseltamivir is recommended as first-line therapy for patients with suspected avian influenza A/H5N1.17
Recommended treatment for other pathogens can be found at http://www.journals.uchicago.edu/CID/journal/issues/v44nS2/41620/41620.tb9.html.
DURATION OF THERAPY
There are no clinical trials that have specifically addressed this issue. Courses as short as 5 days may be sufficient18 but antibiotics should be continued until the patient has been afebrile for 48–72 hours and organ dysfunction has largely resolved.7 Short courses may be suboptimal for patients with bacteraemic Staphylococcus aureus pneumonia, meningitis or endocarditis complicating pneumonia or infection with less common organisms (e.g. Burkholderia pseudomallei or fungi) or Pseudomonas aeruginosa.
RESPONSE TO THERAPY7,9,19
If the patient fails to respond, consider the following questions:
Useful investigations include computed tomography of the chest, bronchoalveolar lavage (Table 32.4) and transbronchial or open-lung biopsy.
Table 32.4 Procedure for obtaining microbiological samples using bronchoscopy and protected specimen brushing (PSB) and/or bronchoalveolar lavage (BAL)12,13
| Infection control | In patients suspected of having a disease which is transmitted by the airborne route (e.g. tuberculosis): • the risk of transmission should be carefully weighed against the benefits of bronchoscopy, which may generate large numbers of airborne particles |
| General recommendations | Suction through the endotracheal tube should be performed before bronchoscopy |
| Avoid suction or injection through the working channel of the bronchoscope | |
| Perform protected specimen brushing before bronchoalveolar lavage | |
| Ventilated patients | Set FiO2 at 1.0 |
| Set peak pressure alarm at a level that allows adequate ventilation | |
| Titrate ventilator settings against exhaled tidal volume | |
| Consider neuromuscular blockade in addition to sedation in patients at high risk of complications who are undergoing prolonged bronchoscopy | |
| Protected specimen brushing | Sample the consolidated segment of lung at subsegmental level |
| If purulent secretions are not seen, advance the brush until it can no longer be seen but avoid wedging it in a peripheral position | |
| Move brush back and forth and rotate it several times | |
| Bronchoalveolar lavage | Wedge tip of bronchoscope into a subsegment of the consolidated segment of lung |
| Inject, aspirate and collect 20 ml of sterile isotonic saline. Do not use this sample for quantitative microbiology or identification of intracellular organisms. It can be used for other microbiological analysis | |
| Inject, aspirate and collect additional aliquots of 20–60 ml | |
| The total volume of saline injected should be 60–200 ml | |
| Complications | Hypoxaemia (possibly less with smaller BAL volumes) |
| Arrhythmia | |
| Transient worsening in pulmonary infiltrates | |
| Bleeding (particularly following PSB) | |
| Fever (more common after BAL) | |
| Positive results | > 5% of cells in cytocentrifuge preparations of BAL fluid contain intracellular bacteria or |
| ≥ 103 colony-forming units/ml in PSB specimen or | |
| ≥ 104 colony-forming units/ml in BAL fluid |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




