CHAPTER 4 Physical Examination in the Cardiac Intensive Care Unit
Vital Signs
Temperature
Because core body temperature is carefully controlled within a narrow range, the detection of hyperthermia or hypothermia offers important clinical clues. Normal oral body temperature is approximately 37° C (98.6° F) with early morning temperatures (approximately 1° C lower) compared with later in the afternoon. By convention, fever is defined as an oral temperature greater than 38° C (>100° F), although it is common practice to consider temperatures greater than 38.4° C (>101.1° F) in hospitalized patients to be clinically significant (albeit without significant data to support this assumption).
Hyperthermia associated with infection (for patients not receiving negative chronotropic agents or with intrinsic cardiac conduction disease) should be accompanied by an increase in the pulse rate of approximately 8.5 beats/min for each 1° C increase in temperature (Liebermeister’s rule).1 The presence of a factitious fever is suggested by the lack of a similar temperature elevation in voided urine compared with the oral temperature. Although a hot drink can quickly increase oral temperature up to 2° C, 5 minutes later the increase is only 0.3° C.2
The presence of hypothermia (oral temperature <35° C (<95° F) requires confirmation. Drinking ice water reduces the oral temperature up to 0.6° C (1° F) for 5 minutes.2 False-negative hypothermic readings can also occur with ear temperatures taken in the presence of cerumen and oral temperatures recorded in the presence of tachypnea. Confirmed hypothermia requires the assessment of a patient’s temperature with a rectal thermometer (which averages approximately 0.6° C (1° F) higher than the oral temperature). The differential diagnosis of true hypothermia includes ambient cold exposure, submersion, hypothyroidism, hypoglycemia, sepsis, and adrenal insufficiency. With hypothermia from submersion or exposure, warming to room temperature is necessary for adequate assessment of end-organ and neurologic function.
Respiration
Tachypnea (>25 breaths/min), when secondary to hypoxia, should nearly always be associated with a reflex tachycardia. Although resting tachypnea may occur with cardiopulmonary disease, it may also be present in response to fever, pain, anemia, hyperthyroidism, abdominal distention, respiratory muscle paralysis, obesity, or metabolic acidosis. When tachypnea accompanies chest pain or collapse, acute pulmonary embolism should be included in the differential diagnosis. When tachypnea is present with a history of orthopnea, it suggests the presence of pulmonary edema, pleural effusion or both. When tachypnea is present in a patient being weaned from a ventilator, tachypnea predicts weaning failure.3
Breathing patterns can reveal underlying pathology (Table 4-1). Exaggerated deep and rapid respirations were noted by Kussmaul to imply the presence of diabetic ketoacidosis because most causes of hypoxia usually result in shallow and rapid respirations. Apneic episodes with snoring suggest obstructive sleep apnea, a potentially treatable contributor to hypertension and right heart failure. Cheyne-Stokes breathing, in which periods of waxing and waning tachypnea and hyperpnea alternate with apnea, occurs in various cardiac, neurologic, and pulmonary disorders or with simple oversedation. When Cheyne-Stokes breathing occurs in the setting of uremia or heart failure, it portends a poor prognosis. Biot breathing is characterized by irregularly irregular breaths of equal depth that are associated with periods of apnea. It can be seen in patients with intracranial disease affecting the medulla oblongata. More severe damage to the medulla oblongata results in ataxic respiration, the complete irregularity of breathing, with irregular pauses and increasing periods of apnea. As this breathing pattern deteriorates further, it merges with agonal respiration.
| Respiratory Pattern | Consider | Eponym/Classification |
|---|---|---|
| Deep and rapid | Diabetic ketoacidosis | Kussmaul respiration |
| Snoring with episodic apnea | Obstructive sleep apnea | |
| Waxing and waning tachypnea/hypopnea alternating with apnea | Oversedation | Cheyne-Stokes breathing |
| Heart failure | ||
| Severe CNS process | ||
| Respiratory failure | ||
| Renal disease (uremia) | ||
| Irregularly irregular (yet equal) breaths alternating with periods of apnea | Damage to the medulla oblongata (intracranial disease) | Biot breathing |
| Completely irregular breaths (pauses with escalating periods of apnea) | Severe damage to the medulla oblongata | Ataxic respiration |
| No breaths or occasional gasps | Severe cardiovascular or neurologic disease | Agonal breathing |
CNS, central nervous system.
Orthopnea (shortness of breath while supine) is most commonly present in patients with heart failure and pleural effusion, but also can occur with ascites, morbid obesity, and diaphragmatic paralysis. Alternatively, platypnea (shortness of breath when assuming the upright position) suggests the right-to-left shunting that occurs with an atrial septal defect or intrapulmonary shunt. Trepopnea (shortness of breath while lying on one side) occurs with a right pleural effusion or with unilateral lung or diaphragm disease when the healthy lung is down.4,5
Pulse
The pulse should be assessed bilaterally for presence, rate, volume, contour, and regularity. An initial examination should always contain a description of the radial and carotid arteries, in addition to the brachial, femoral, popliteal, and pedal pulses. This examination is important for patients with hypotension, claudication, arterial insufficiency, or cerebrovascular accident, or after intra-aortic balloon pump insertion. Assessing the pulse for 30 seconds is more accurate than counting for only 15 seconds.6
A discrepancy in bilateral upper extremity pulses (especially with decreases in rate or volume on the left side) raises the possibility of aortic dissection, subclavian narrowing secondary to atherosclerosis or congenital webs. If such a discrepancy is present, the examiner should search for evidence of a subclavian steal phenomenon, detected as a decrease in pulse amplitude after raising or exercising the affected arm for approximately 45 seconds (the left side is affected 70% of the time; the reduction in systolic blood pressure is >20 mm Hg 94% of the time).7 Aortic dissection is suggested by the presence of a pulse deficit, focal neurologic signs, and mediastinal widening on the chest radiograph.8 Diminished lower extremity pulses are consistent with coarctation of the aorta or atherosclerotic disease of the abdominal aorta. Although the detection of low femoral pulse amplitude (or its absence) is crucial for assessing the risk-to-benefit ratio in patients who may require vascular access or device implantation, its diminution or absence after catheterization or intra-aortic balloon pump implantation requires urgent intervention.
When tachycardia (a heart rate >100 beats/min) is present, the regularity of the rhythm offers important diagnostic clues. Regular rhythm rates between 125 beats/min and 160 beats/min suggest sinus tachycardia, the presence of atrial flutter with 2:1 block, or ventricular tachycardia. The presence of intermittent cannon A waves in the neck veins is highly sensitive, whereas a changing intensity of the first heart sound (S1) is highly specific for the detection of ventricular tachycardia.9 Atrial flutter may be accompanied by rapid undulations in the jugular venous pulse (flutter waves or F waves). Because sinus tachycardia may be due to correctable causes, such as hypovolemia, hypoxia, infection, hyperthyroidism, anemia, or anxiety, or may be due to the pathologic adaptation occurring with chronic heart failure or myocardial ischemia, integration of these clinical suspicions with the nature of the underlying rhythm is important. The use of vagal maneuvers may help differentiate the causes of narrow-complex tachycardia.
The Valsalva maneuver, performed by asking the patient to bear down as if “having a bowel movement” or pushing up the abdomen against the examiner’s hand placed on the middle of the abdomen, seems to be more effective than carotid sinus massage, performed by pressing on the neck at the bifurcation of the carotid artery just below the angle of the jaw, at terminating supraventricular tachycardia10,11 in 50% of cases. Paroxysmal supraventricular tachycardia (nodal re-entry and reciprocating tachycardias) may be interrupted with enhanced vagal tone. Sinus tachycardia, atrial flutter, and atrial fibrillation usually slow only transiently (but may reveal the underlying rhythm), although an abrupt halving of the rate may occur with atrial flutter. Detection of an irregular tachycardia on physical examination suggests atrial fibrillation, atrial premature beats, or ventricular premature contractions. In atrial fibrillation, assessment of the apical rate (counting heartbeats via auscultation) is more accurate than counting the radial pulse, accounting for a “pulse deficit.”12
Bradycardia (heart rate <50 beats/min) may be appropriate in trained athletes, but should be asymptomatic and associated with a gradual increase in heart rate with exercise.13 Detection of a regular-rhythm bradycardia in a patient with fatigue, mental status changes, or evidence of impaired peripheral perfusion or pulmonary congestion raises the possibility of pharmacologic toxicity (digoxin, β blockers, or calcium channel blockers), hypothermia (owing to hypothyroidism or exposure), or an atrioventricular nodal or ventricular escape rhythm that occurs with complete heart block or sick sinus syndrome.
Appreciation of the pulse volume and contour is also informative (Table 4-2). Tachycardia with a bounding pulse is present with septic shock (owing to the acute reduction in afterload), hyperthyroidism, or the sudden collapse of the pulse with chronic aortic insufficiency (a water-hammer pulse). Consistent with the presence of chronic aortic insufficiency is the accentuation of the radial pulse when the examiner lifts the whole arm above the patient’s head (Mayne’s sign). A weak and thready pulse may be present with severe left ventricular dysfunction, hypovolemia, severe mitral regurgitation, or complete heart block. A slow rising and weak carotid pulse (pulsus parvus et tardus) is consistent with a diagnosis of severe aortic stenosis, whereas a regular pulse that alternates between weak and strong (pulsus alternans) occurs with left ventricular dysfunction or pericardial tamponade. A “double tap” during systole (pulsus bisferiens) can occur with either hypertrophic cardiomyopathy or the combination of aortic stenosis and aortic insufficiency.14,15 In the presence of a bisferiens pulse, two soft and rapid sounds can be auscultated with each cardiac cycle as the brachial artery is compressed by a blood pressure cuff proximally.16
Table 4–2 Pulse Characteristics
| Pulse Description | Consider |
|---|---|
| Bounding | Septic shock, hyperthyroidism, chronic AI |
| Weak and thready | Severe LV dysfunction, hypovolemia, severe MR, complete heart block, pericardial effusion |
| Slow rising and weak | Severe AS |
| Alternating between strong and weak | LV dysfunction, pericardial tamponade |
| Double tap (pulsus bisferiens) | Hypertrophic cardiomyopathy, AS with AI |
AI, aortic insufficiency; AS, aortic stenosis; LV, left ventricular; MR, mitral regurgitation.
Blood Pressure
In the ICU, there is no rule defining “normal blood pressure.” Adequate blood pressure varies by patient and clinical status, but is generally believed to consist of a mean perfusion pressure of at least 60 mm Hg and the absence of end-organ hypoperfusion. For accurate assessment, an adequately sized blood pressure cuff must be used (there are lines on all blood pressure cuffs to indicate adequate sizing) and should be correctly situated around the bicep (and not over clothing). A blood pressure obtained with a cuff that is too short or narrow, especially if the patient is obese or has an enlarged upper arm, may result in a factitiously elevated blood pressure.17,18
Although palpation of pulses is commonly used in emergency situations to estimate systolic blood pressure (i.e., palpation of a radial pulse suggests a minimum systolic blood pressure of 80 mm Hg; a femoral pulse, a blood pressure of at least 70 mm Hg; and a carotid pulse, a blood pressure of at least 60 mm Hg), the overall accuracy of this estimation has been questioned.19 To obtain the palpable systolic blood pressure, the cuff should first be inflated until the radial pulse is no longer palpable (usually 150 to 200 mm Hg) and then slowly deflated (2 to 3 mm Hg per second) until the pulse returns.
Hypotension without a concomitant increase in the pulse rate (in the absence of medications that can blunt a heart rate response) raises the possibility of autonomic dysfunction. The presence of a pulsus paradoxus (a >10 mm Hg decrease in systolic blood pressure occurring at end-expiration with the patient breathing normally) can occur with cardiac tamponade (very sensitive when occurring with tachycardia, jugular venous distention, and an absent y descent),20,21 constrictive pericarditis (occurring with jugular venous distention that persistently augments with inspiration, a pericardial knock, hepatomegaly, and an exaggerated y descent),22 severe hypertension, pulmonary embolism, COPD, and severe obesity.
With appropriate clinical scenarios, blood pressure should also be assessed in both arms and one leg. Leg blood pressure can be assessed by placing the blood pressure cuff around the calf and using the dorsalis pedis pulse for auscultation or Doppler interrogation. A systolic blood pressure difference greater than 10 mm Hg between arms suggests aortic dissection, proximal aortic aneurysm, or subclavian artery stenosis. With coarctation of the aorta, arm blood pressures are greater than blood pressures in the legs (this may also be accompanied by underdeveloped lower extremity musculature compared with upper extremity musculature). Leg blood pressure that is more than 15 mm Hg higher than arm blood pressure suggests aortic dissection, aortic insufficiency, or a proximal vasculitis (i.e., giant cell or Takayasu arteritis).
Head, Eyes, Ears, Nose, and Throat Examination
Eyelid xanthelasma or a corneal arcus or both may occur with either hypercholesterolemia or diabetes mellitus. Yellowed sclera are seen with hyperbilirubinemia, whereas blue sclera can be seen in Marfan and Ehlers-Danlos syndromes. Dry, puffy, and sunken (enophthalmic) eyes are consistent with hypothyroidism, whereas exophthalmic eyes with lid lag (white sclera visible between the margin of the upper eyelid and the corneal limbus with the patient looking downward) associated with a lid lag (an immobility or lagging of the upper eyelid on downward rotation of the eye) and lid retraction (widening of the palpebral fissure) are associated with hyperthyroidism. Periorbital edema is seen with the hypoalbuminemia of hepatic disease, a protein-losing nephropathy, or the superior vena cava syndrome. The lack of periorbital edema with diffuse peripheral edema is a distinguishing feature of a cardiac versus hepatic or renal cause of peripheral edema; it is due to the inability of patients with heart failure and severe volume overload to elevate their upper torso to breathe more comfortably. Conjunctival pallor is a very specific sign of anemia, and this diagnosis is reinforced by the presence of concomitant palmar and palmar crease pallor.23
When firmly palpating the patient’s thyroid gland with the neck flexed (to relax the sternohyoid and sternocleidomastoid muscles), significant findings can include an enlarged thyroid (size appreciated larger than an inch) and the presence of nodules (4% prevalence; most are benign). It is important to note the size and site of these nodules for follow-up examinations.24 During swallowing, the thyroid gland rises upward with the trachea to allow location of a neck mass either within or outside the thyroid gland.
Jugular Venous Pulse and Abdominojugular Reflux
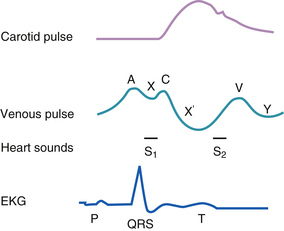
The internal jugular venous pulse (JVP) is useful manometer for right atrial pressure. However, it is only accurate in indicating intravascular volume status and pulmonary capillary wedge pressure in the absence of tricuspid stenosis, right ventricular dysfunction, pulmonary hypertension, and a restrictive or constrictive cardiomyopathy. The JVP should be sought by first asking the patient to lift their chin up and turn to the left against the resistance of the examiner’s right hand. Within the triangle formed by the visible heads of the sternocleidomastoid muscle and the clavicle, the examiner should then search with the neck muscles relaxed, for the weak impulses of the jugular vein along a line from the jaw to the clavicle. Shining a tangential light from slightly behind the neck can accentuate the visibility of the transmitted venous impulses. Simultaneous palpation of the radial pulse, assuming the patient is in sinus rhythm, allows detection of a neck pulsation (a wave) immediately preceding the peripheral pulse (Fig. 4-1). Alternatively, one can visualize the x descent as an inward movement along the line of the jugular vein that occurs simultaneously with the peripheral pulse.
In patients with presumed volume overload, jugular venous distention may be best assessed with the patient sitting upright at 90 degrees, a position in which the clavicle is approximately 7 to 8 cm above the right atrium (equivalent to the upper limit of normal for right atrial pressure, 5 to 7 mm Hg). The 7 to 8 cm is added to the maximal vertical distance at which any venous pulsations are seen above the clavicle to estimate the right atrial pressure. If the JVP cannot be appreciated in the upright position, an attempt can be made to visualize it sequentially with the upper body at a 45-degree angle (where only 4 to 5 cm is needed to the distance above the clavicle where the venous pulsations were seen). If venous pulsations are still difficult to discern, either of two extremes may be present: either the lack of elevation of the right atrial pressure or jugular venous distention above the angle of the jaw, even in the upright position.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree