ANTIBACTERIAL DRUGS
Effective antibacterial drugs can either inhibit the growth of (bacteriostatic) or kill (bactericidal) bacteria. Antibacterial effects result from the inhibition of cell wall synthesis, inhibition of intrabacterial protein synthesis, alteration in nucleic acid metabolism, or intrabacterial enzyme inhibition (Table 163-1). The drug mechanism of action does not necessarily correlate with bacteriostatic or bactericidal effects, because the latter are affected also by the concentration of antibiotic to which bacteria are exposed. Drugs of choice for most infections are not based on a bacteriostatic or bactericidal effect of an agent, but rather are chosen based on whether the drug reaches the site of infection in adequate quantities, the spectrum of the agent, its safety, and cost.
| Cell wall active agents | Nucleic acid inhibitors |
| Penicillins | Fluoroquinolones |
| Vancomycin | Rifampin |
| Cephalosporins | Nitrofurantoin |
| Teicoplanin | Enzyme inhibitors |
| Telavancin | Fosfomycin |
| Daptomycin | Sulfonamides |
| Colistin | Trimethoprim |
| Polymyxin B | |
| Protein synthesis inhibitors | |
| Aminoglycosides | |
| Macrolides | |
| Linezolid | |
| Tetracyclines (including tigecycline) | |
| Clindamycin | |
| Quinupristin/dalfopristin |
MECHANISMS OF ACTION
β-Lactam (penicillins, cephalosporins) and glycopeptide antibiotics (vancomycin, telavancin, teicoplanin) bind to receptors in the bacterial cell wall. The target receptors for penicillins and cephalosporins are called penicillin-binding proteins. Autolytic enzymes within the cell wall bind to penicillin-binding proteins; once activated, the enzymes damage the peptidoglycan component of the cell wall, creating weakening and eventual cell lysis. Glycopeptide antibiotics bind to a terminal dipeptide (alanine-alanine) in the cell wall peptidoglycan and prevent the necessary cross-linking for a competent cell wall structure. At usual doses, β-lactam and glycopeptide antibiotics are bactericidal. Resistance arises due to mutations in the penicillin-binding proteins, leading to reduced β-lactam binding (e.g., by oxacillin-resistant Staphylococcus aureus or penicillin-resistant Streptococcus pneumoniae) or changes to the terminal dipeptide (e.g., by vancomycin-resistant Enterococcus faecium) that reduce the level of binding. Daptomycin inserts a lipophilic part of the molecule into the cell wall of gram-positive bacteria, depolarizing the cell wall, which causes the leakage of intracellular content and a bactericidal effect.
The emergence of multidrug-resistant organisms (most commonly in species of Pseudomonas, Acinetobacter, and Klebsiella) has led to the renewed use of older, but more toxic drugs such as colistin and polymyxin B. These agents interact with the lipids within the cell wall, increasing cell wall permeability, which leads to a bactericidal effect because of the leakage of intracellular contents.
Several classes of antibacterial drugs bind to ribosomes within bacteria, blocking necessary protein synthesis. Aminoglycosides and tetracyclines (including tigecycline) bind to the 30S ribosomal subunit, whereas macrolide antibiotics and clindamycin bind to the 50S subunit. Ribosomal binding inhibits transfer RNA function, decreasing the amount of protein synthesis. Ribosomal-binding drugs enter through the cell wall and bind in adequate concentrations to reversibly inhibit protein synthesis. Resistance mechanisms arise with reduced cell wall permeability, an active efflux pump that removes the antibiotic from the cell, or ribosomal-binding site mutations that decrease antibiotic affinity.
Fluoroquinolone antibiotics inhibit DNA gyrase, the enzyme responsible for DNA unwinding for transcription and recoiling during bacterial replication. Fluoroquinolones must reach the nucleus of the bacterial cell to provoke these effects; resistance can arise when cell wall permeability is reduced, active efflux occurs, or a DNA gyrase mutation has arisen that reduces fluoroquinolone binding. Rifampin is a broad-spectrum antimicrobial agent active against many gram-positive and gram-negative bacteria and mycobacteria. Rifampin (or rifampicin) inhibits RNA synthesis by binding to DNA-dependent RNA polymerase, thereby blocking the initiation of RNA chain formation. Nitrofurantoin is modified by bacterial metabolism to a compound that damages DNA. Susceptible bacteria rarely become resistant to nitrofurantoin.
Sulfonamides and trimethoprim block sequential steps in the formation of folic acid. Sulfonamides inhibit dihydropteroate synthase, the enzyme that converts p-aminobenzoic acid to dihydrofolic acid; trimethoprim inhibits dihydrofolate reductase, the enzyme that converts dihydrofolic to tetrahydrofolic acid. Together, this paired action is an effective bactericidal process and not far removed from the intracellular actions of methotrexate. Fosfomycin inactivates enolpyruvate transferase, inhibiting cell wall synthesis; it is gaining resurgent popularity for single-dose treatment (3 grams orally) of uncomplicated urinary tract infections given the activity against the common pathogens involved. Resistance to these drugs arises by enzyme mutations that reduce the affinity of sulfonamide, trimethoprim, or fosfomycin to their respective enzyme targets.1 These antibacterial drug mechanisms of action are summarized in Figure 163-1. Table 163-2 summarizes the classification, names, and routes of the most common antibiotics within each antibiotic class.1
| Penicillins | |||||||
|---|---|---|---|---|---|---|---|
| Natural Penicillins | Aminopenicillins | Penicillinase-Resistant Penicillins | Antipseudomonal Penicillins | β-Lactam/β-Lactamase Inhibitor Combination | Monobactams and Carbapenems | Aminoglycosides | Fluoroquinolones |
| Penicillin G IV, PO | Ampicillin IV, PO | Oxacillin (Bactocill®) IV, PO | Piperacillin IV | Amoxicillin-clavulanic acid (Augmentin®) PO | Aztreonam (Azactam®) IV | Amikacin IV | Moxifloxacin (Avelox®) IV, PO |
| Penicillin V PO | Amoxicillin (Amoxcil®) PO | Dicloxacillin PO | Ticarcillin-clavulanic acid (Timentin®) IV | Ertapenem (Invanz®) IV | Gentamicin IV | Ciprofloxacin (Cipro®) IV, PO | |
| Nafcillin (Nallpen®) IV, PO | Ampicillin-sulbactam (Unasyn®) IV | Meropenem (Merrem®) IV | Neomycin (Neo-Fradin®) PO | Levofloxacin (Levaquin®) IV, PO | |||
| Piperacillin-tazobactam (Zosyn®) IV | Imipenem (Primaxin®) IV | Streptomycin IM, IV | Gemifloxacin (Factive®) PO | ||||
| Doripenem (Doribax®) IV | Tobramycin IV | ||||||
| Cephalosporins | |||||||
| First Generation | Second Generation | Third Generation | Fourth/Fifth Generation | Macrolides/Tetracyclines | Enzyme Inhibitors | Miscellaneous | |
| Cefazolin (Ancef ®) IV | Cefaclor PO Cefotetan IV | Ceftibuten (Cedax®) PO | Cefepime (Maxipime®) IV | Erythromycin (Erythrocin®) IV, PO | Trimethoprim-sulfamethoxazole (Bactrim®, Septra®) IV, PO | Clindamycin (Cleocin®) IV, PO | |
| Cefadroxil PO | Cefuroxime axetil (Ceftin®) PO | Cefotaxime (Claforan®) IV | Ceftaroline (Teflaro®) IV | Azithromycin (Zithromax®) IV, PO | Trimethoprim PO | Metronidazole (Flagyl®) IV, PO | |
| Cephalexin (Keflex®) PO | Cefprozil PO Cefoxitin (Mefoxin®) IV | Ceftazidime (Fortaz®, Ceptaz®, Tazicef ®) IV | Clarithromycin (Biaxin®) PO | Fosfomycin (Monurol®) PO | Nitrofurantoin (Macrodantin®, Macrobid®) PO | ||
| Cefuroxime (Zinacef ®) IV | Ceftriaxone (Rocephin®) IV | Tetracycline PO | Quinupristin/dalfopristin (Synercid®) IV | ||||
| Cefpodoxime PO | Minocycline (Minocin®) PO | Vancomycin (Vancocin®) IV, PO | |||||
| Cefdinir PO | Doxycycline (Vibramycin®) IV, PO | Linezolid (Zyvox®) IV, PO | |||||
| Cefixime (Suprax®) PO | Tigecycline (Tygacil®) IV | Daptomycin (Cubicin®) IV | |||||
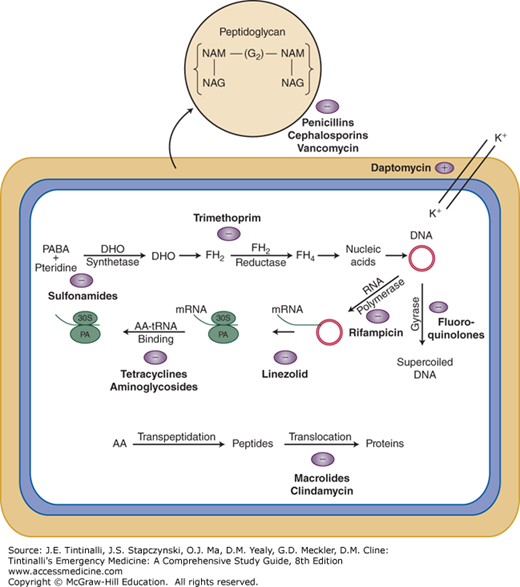
FIGURE 163-1.
Mechanisms of action of antibacterial drugs. The peptidoglycan layer in the bacterial cell wall is a crystal lattice structure formed from linear chains of two alternating amino sugars, namely N-acetylglucosamine (GlcNAc or NAG) and N-acetylmuramic acid (MurNAc or NAM). Penicillins, cephalosporins, and vancomycin are cell wall active agents, preventing the necessary cross-linking within the peptidoglycan layer, rendering it incompetent. Other listed antibiotics exert their actions on cellular mechanisms within the bacteria as shown. AA = amino acids; DHO = dihydropteroate; FH2 = dihydrofolate, FH4 = tetrahydrofolate; (G2) = glucose; K+ = potassium; PA = peptide donor-acceptor site; PABA = p-aminobenzoic acid; + = enhance; – = inhibit.
INDICATIONS IN THE ED AND DRUGS OF CHOICE
Drugs of choice for specific infections are based on clinical effectiveness and adverse events. Successful effectiveness is based on the knowledge of the likely bacterial pathogen responsible for a specific infection type and the usual antimicrobial spectrum of antibiotics. Alternate drugs of choice are selected in cases of resistance to an initial drug, a history of intolerance or allergy to the drug of choice, or because of a higher risk of adverse events. Taking into account those infections most likely to be present in ED patients and the most likely pathogens involved in these infections, Table 163-3 summarizes drugs of choice for common infections.2
| Site/Type of Infection | Suspected Organisms | Drug of Choice | Alternative |
|---|---|---|---|
| Respiratory | |||
| Pharyngitis | Group A streptococci | Penicillin V | Macrolide |
| Bronchitis,* otitis,* acute sinusitis* | Streptococcus pneumoniae Haemophilus influenzae | Amoxicillin, amox/clav, or cefuroxime | Macrolide or doxycycline |
| Epiglottitis | H. influenzae, Group A streptococci | Ceftriaxone | Cefuroxime |
| Community-acquired pneumonia | |||
| Normal host | S. pneumoniae, viral, Mycoplasma | Azithromycin or doxycycline | Levofloxacin |
| Aspiration | Aerobes and anaerobes | Clindamycin | Pip/TZ, ceftriaxone plus metronidazole |
| Alcoholic | S. pneumoniae, Klebsiella | Ceftriaxone | Levofloxacin |
| Urinary tract infection | Escherichia coli and other enteric gram-negative rods | TMP/SMX† | Ciprofloxacin, cephalexin, nitrofurantoin, or fosfomycin (latter single dose 3 grams PO) |
| Sexually transmitted infections | |||
| Urethritis | Neisseria gonorrhea, Chlamydia | Ceftriaxone, azithromycin | Cefixime, doxycycline |
| Genital ulcers | Treponema pallidum, herpes simplex virus | Penicillin G Acyclovir | Doxycycline Valacyclovir |
| Skin/soft tissue | |||
| Cellulitis | Group A streptococci, Staphylococcus aureus | Cephalexin† | Dicloxacillin, clindamycin, TMP/SMX, or vancomycin |
| Necrotizing fasciitis | Polymicrobial | Imipenem or meropenem Plus vancomycin | — |
| Fresh/brackish water infections | Mixed flora, Aeromonas | TMP/SMX | Fluoroquinolone |
| Cat bite | Pasteurella, mixed flora | Amox/clav | Clindamycin and ciprofloxacin |
| Meningitis | |||
| Normal host | S. pneumoniae, Neisseria meningitidis, S. aureus | Ceftriaxone and vancomycin | — |
| Immunocompromised or >50 y old | Listeria, H. influenzae | Add ampicillin | — |
| Acute abdomen (perforation) | Gram-negative rods, anaerobes, enterococci | Ampicillin/sulbactam or Pip/TZ | Cefoxitin or cefotetan or imipenem |
ANTIBIOTIC DOSAGE AND DOSAGE ADJUSTMENTS
Adequate drug dosage takes into account achievable serum and tissue levels plus the concentrations necessary (determined in the laboratory) to inhibit the growth of susceptible bacteria. Standard dosing guidelines usually result in successful treatment when a susceptible organism is present and barriers to drug penetration (i.e., abscess) are absent. If antibiotic penetration issues exist, such as in suspected meningitis, endocarditis, or osteomyelitis, give the highest doses recommended to improve effect.
Dosage adjustments of many antibiotics are necessary for patients with renal disease to prevent adverse events from drug accumulation, most notably when using IV administration. Oral doses are typically lower, so toxic drug accumulation is less likely in those with renal dysfunction. Dosage modifications in liver disease are less clear because of limited ways to accurately assess decreases in drug elimination characteristics or rate. Fosfomycin in single-dose use for urinary tract infection requires no adjustment. Guidelines for dosing adjustment, primarily with IV therapy, are summarized in Table 163-4.3