Fig. 12.1
Probability of survival according to the PaO2/FiO2 ratio in the ACURASYS study (Reproduced with permission from Papazian et al. [16], Appendix 2)
12.4 Summary of the Evidence-Based Data and Limitations
Two recent meta-analyses based on these three trials [17, 18] have investigated the role of NMBA use for ARDS. The meta-analysis conducted by Alhazzani and colleagues [17] concluded that the use of cisatracurium besylate for a short period in the early phase of ARDS consistently reduced the risk of death at 28 days, reduced the times to ICU discharge and hospital discharge (number needed to treat = 9), reduced the risk of barotrauma, increased the number of ventilator-free days and did not affect the risk of ICU-acquired weakness. The study conducted by Neto and colleagues [18] strongly confirmed all of these results and stated that the use of NMBAs was associated with decreases in the PEEP and plateau pressure over time in the group of paralysed patients, which reflected improved ventilation.
The main criticism that could be levied is that these two meta-analyses were based on only three randomized controlled trials that were conducted by the same group, which may have introduced bias into the conclusions. However, as noted by Neto and colleagues, the main study on which these meta-analyses were based included 20 different ICUs, which reduced the risk of bias.
12.5 Physiopathology
12.5.1 Short-Term Effects of NMBAs
The majority of the studies that have reported the effects of NMBA-induced paralysis were conducted in very short periods of time (<24 h) during anaesthesia (i.e. in “normal lungs”) with muscle paralysis and mechanical ventilation without PEEP [19–21]. Tokics and colleagues demonstrated that patients displayed atelectasis. Shunt was placed in gravity-dependent lung regions that corresponded to the atelectatic areas. There were considerable V˙/Q˙ ventilation-to-perfusion mismatches that resulted in ventilation primarily of the ventral lung regions and perfusion of the dorsal lung regions [20]. Hedenstierna and colleagues demonstrated that due to the reduction of the cross-sectional chest area and the cephalic ascension of the diaphragm, the thoracic volume was reduced. This reduction was accompanied by a decrease in the functional residual capacity (FRC) that was attributed (at least in part) to the loss of muscular tone [21, 22] and was thought to be a major cause of post-operative hypoxaemia. However, few studies have reported the effects of anaesthesia and NMBA-induced paralysis on lung mechanics. In healthy subjects, Brismar and colleagues found no further reduction in FRC or modification of the elastic properties of the lung or the chest wall following the addition of paralysis to anaesthesia [23]. In the intensive care setting, Conti and colleagues conducted a study of 13 patients affected by diseases involving both lungs and the chest wall who required mechanical ventilation for acute respiratory failure and were heavily sedated (Ramsay scores of 5) and failed to demonstrate any further modification of either the chest wall or lung elastance following paralysis. CT scans and analysis of the pressure-volume curves revealed that derecruitment was reduced after the application of a PEEP of 10 cmH2O [21, 24]. In summary, no data support any additional deleterious role of NMBAs in the onset of atelectasis in sedated ARDS patients.
12.5.2 Pathophysiological Hypothesis of the Beneficial Effects of NMBAs
The results of the three available RCTs about the use of NMBAs during the early phase of ARDS indicate that the effect of the treatment on oxygenation becomes significant after 24 h. Moreover, in the ACURASYS trial, the Kaplan-Meier survival curves did not separate until 18 days of treatment. These observations together with the available knowledge regarding the use of NMBAs for shorter periods in patients with healthy lungs raise hypotheses regarding the manners in which NMBAs could be beneficial (Fig. 12.2) during the acute phase of severe ARDS. Several mechanisms may be involved and are most likely interrelated. Slutsky [25] proposed a summary of the effects of NMBAs that included the following main points:
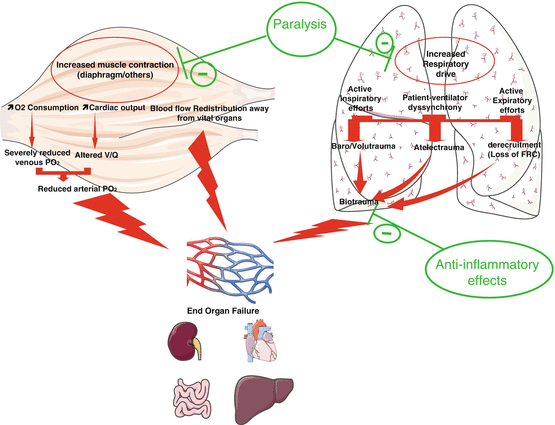
Patient-to-ventilator dyssynchronies are reduced, and the control of tidal volume is improved, which leads to decreases in baro- and volutrauma as well as a decrease in atelectrauma due to the inhibition of active expiration and the improved control of the PEEP. This latter effect is associated with decreases in lung blood flow and alveolar-capillary permeability.
NMBA use is associated with a decrease in respiratory drive that is classically associated with hypoxaemia and permissive hypercapnia.
NMBA use leads to a decrease in biotrauma, an inhibition of the translocation of inflammatory mediators from the alveolar space to the circulation and a decrease in associated organ failure. These suppositions are supported by the decreased production of pro-inflammatory cytokines in both the lung and blood observed by Forel et al. [15]. Moreover, a direct anti-inflammatory role of cisatracurium via nicotinic acetylcholine receptor-α 1 has recently been demonstrated in in vitro and murine models [26].
NMBA use results in a progressive increase in the functional residual capacity and a decrease in intrapulmonary shunting. The improvement in the ventilation-perfusion ratio may also be related to the more uniform distribution of the pulmonary perfusion due to the application of lower pulmonary pressure, which favours the perfusion of ventilated areas and decreases intrapulmonary shunting.

Fig. 12.2
Potential physiopathological effects that explain the benefits of NMBAs in the most severe forms of ARDS. V/Q ventilator/perfusion ratio, FRC functional residual capacity
12.5.3 Deleterious Effects of Spontaneous Breathing in the Acute Phase of ARDS
The beneficial effects of NMBAs in the most hypoxaemic patients in the initial phase of ARDS may also be due to the prevention of the deleterious role of spontaneous breathing (SB). In the early phase of ARDS, the high respiratory drive that results from hypoxaemia may result in elevated and uncontrolled transpulmonary pressure (TPP) with over-distension, particularly in the dependant vertebro-diaphragmatic zones, which results in major ventilator-induced lung injury (VILI) [30]. In these cases, the maintenance of a plateau pressure below 30 cmH2O might not be sufficient to ensure protective ventilation, particularly in patients with the most severe forms of ARDS, as suggested by recent experimental work from Yoshida et al. [31]. In a rabbit model of ARDS, these authors demonstrated that the preservation of ventilatory effort lacked the same effects on oxygenation, pulmonary ventilation and lung injury depending on the severity of the ARDS (i.e. mild or severe). The preservation of SB induced improvements in pulmonary ventilation and oxygenation in mild ARDS subjects but resulted in increased TPP and VILI in a group of animals with severe ARDS. These deteriorations of the ventilatory and histological parameters were prevented by abolishing SB via the administration of NMBAs in the severe ARDS group.
In summary and in contrast to what occurs in patients with mild to moderate ARDS, in the initial phase of severe ARDS, the prevention of VILI and the optimization of alveolar recruitment appear to be based on controlled protective ventilation and the use of NMBAs and the consequent abolition of spontaneous ventilatory effort.
12.6 Adverse Events
12.6.1 NMBAS and ICU-Acquired Weakness (ICUAW)
NMBAs have been dispraised due to a supposed association with ICUAW.
ICUAW is defined by a generalized muscle weakness that develops during the course of an ICU admission and for which no cause other than the acute illness or its treatment can be identified [32]. ICUAW is present among approximately 60% of ARDS patients at the time of awakening [33] and 36% at the time of hospital discharge [34]. ICUAW is associated with prolonged ICU and hospital stays, prolonged mechanical ventilation duration and increased ICU and hospital mortalities [35–38]. Risk factors have been discussed widely in the literature, and independent risk factors, such as female sex, multiple organ dysfunctions (≥2), the duration of mechanical ventilation, hyperglycaemia [39] and the administration of corticosteroids [36], have been identified.
The prolonged use of neuromuscular blocking agents (more than 48 h) [40, 41], their simultaneous administration with corticosteroids [42] and steroid NMBAs may contribute to ICUAW [43, 44], but there is actually no evidence that non-steroid NMBAs increase the risk of ICUAW when used for short durations and without the simultaneous administration of corticosteroids [32]. In a meta-analysis [17] of RCTs evaluating the effects of the use of NMBAs during ARDS, the incidence of ICU-acquired paresis (assessed via clinical evaluations of quadriparesis or by the Medical Research Council (MRC) score at day 28 or at ICU discharge) was not higher among the treated patients than in the control group.
12.6.2 Insufficient Sedation and Memorizing
The continuous use of NMBA infusion highlights the problem of inadequate sedation. Hardin et al. [45] demonstrated that patients receiving NMBAs were awake for 22% of the sleep period over a time span of 24 h. Neuromonitoring via continuous electroencephalography or with a device such as the bispectral index (BIS, Aspect Medical Systems, Natick, MA, USA) can reduce the risk of consciousness in paralysed patients [46]. However, studies evaluating the reliabilities of such devices in the monitoring of the level of sedation of paralysed patients have found contradictory results [47, 48].
12.7 Perspectives: The Appropriate Place of NMBAs in ARDS Treatment
Clear recent recommendations concerning the use of NMBAs for ARDS are lacking. The most recent guidelines from 2002 [1] reduce the role of NMBAs to “facilitating mechanical ventilation when sedation alone is inadequate”. These guidelines were created before the RCTs were conducted and appear to be obsolete. The recent data on the beneficial effects of NMBAs on mortality have modified their use. In a recent large international survey conducted in 50 countries, NMBAs were found to be the most frequently used adjunctive measure in severe ARDS [7]. NMBAs were also extensively used in the PROSEVA study, which demonstrated the beneficial effect of prone positioning on mortality [6]. Overall, that NMBAs have a crucial role in the management of ARDS seems to no longer be questionable. However, the available literature conveys the following “rules” with respect to the use of NMBAs:
First, NMBAs should be used to paralyse patients in the early phase of the evolution of ARDS.
Second, short courses of treatment should be administered to limit the occurrence of ICUAW. Forty-eight-hour treatments were used in the ACURASYS study [16], but shorter treatments (24 h) might be sufficient if the evolution is rapid and favourable. In contrast, in the most severe cases, a longer period may be required (72–96 h), particularly for patients who require several days of PP (as in the PROSEVA study) and those who require extracorporeal life support.
Third, NMBAs should be reserved for the most hypoxaemic patients (including severe ARDS and the most hypoxaemic cases of moderate ARDS). A PaO2/FiO2 ratio cut-off of 120 can be proposed based on the ACURASYS study data [16], but a ratio of 150 might also be used. This cut-off is proposed in the most recent guidelines of the Surviving Sepsis Campaign, which support the use of short-course treatment with NMBAs during sepsis-related ARDS [49]. Moreover, NMBAs are almost always associated with PP [6], and PP has been demonstrated to be beneficial for patients with a PaO2/FiO2 ratio below 150. The Reevaluation Of Systemic Early Neuromuscular Blockade (ROSE study NCT02509078) study is currently recruiting patients with PaO2/FiO2 ratios below 150 and will hopefully help to form a conclusion.
Fourth, NMBAs should be integrated into ventilatory strategies that allow for spontaneous ventilation as soon as the ventilatory parameters improve (Fig. 12.3). Indeed, in the ACURASYS study, following the initial 48-h period, NMBAs were discontinued, sedatives were reduced and pressure support ventilation was introduced in all patients with FiO2 values less than or equal to 0.6. This concept was also present in the PROSEVA study; in this trial, when the PaO2/FiO2 ratio was ≥150 mmHg, the PEEP was ≤10 cmH2O and the FiO2 was ≤60%, the PP sessions, sedation and paralysis were stopped to allow for spontaneous efforts.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





