Fig. 12.1
Data on subscales of the Multidimensional Pain Inventory (MPI) for amputees suffering exclusively from phantom limb pain (PLP, N = 190), residual limb pain (RLP, N = 100), or both (PLP + RLP, N = 191). For comparison, data from a sample of chronic back pain patients (CBP, N = 101) as well as norm MPI data of a sample of 250 patients with chronic musculoskeletal pain (MSP) are given. Error bars indicate standard error of the mean (SEM). The age of amputees and CBP patients ranged from 18 to 70, and the mean duration of pain presence in these samples was more than 10 years. Chronic post-amputation pain sufferers reported less pain, pain-related interference, and affective distress and more life control compared to CBP sufferers. The only exception is the social support subscale, which is similar to CBP patients in RLP sufferers (exclusively or in combination with PLP) (p < .05, Bonferroni corrected for multiple t-tests)
12.4.2 Anxiety
Anxiety is generally discussed as a factor associated with the aggravation and maintenance of chronic pain, related to avoidance of activity and perceived disability (Turk 2002). As it is the case for depression, anxiety symptoms are common immediately following amputation (Shukla et al. 1982a; Singh et al. 2009) but, in that early stage, do not chiefly concern PLP. In their review, Horgan and MacLachlan (2004) state that anxiety following amputation commonly relates to a changed body image, social functioning and social discomfort, and adaptation to a new identity. However, they also state that too few empirical data exist about the process of adaptation of the amputees’ identity to being disabled and concomitant anxiety themes. Rather than being associated with PLP, anxiety is correlated with somatic symptoms (e.g., exhaustion, insomnia) in the early phase after amputation (Whyte and Niven 2011a). Similar to the course of depression, anxiety levels decline within the first years (Horgan and MacLachlan 2004; Singh et al. 2009). Little is known about the association of anxiety and PLP in a later stage, after the consequences of an acute amputation have subsided. Desmond and MacLachlan (2006) found that anxiety was higher in long-term amputees with chronic pain (PLP or residual limb pain) compared to pain-free amputees, but anxiety scores were within the range of the normal population. As mentioned above, their sample also consisted only of former military service members with traumatic limb loss. Castillo et al. (2013) showed that in a late, chronic phase of pain following lower-extremity trauma, anxiety—not depression—predicted subsequent pain. However, their sample was heterogeneous and contained amputees and non-amputees, and no distinction was made between PLP and other types of pain. Finally, fear of pain and movement have been identified as important predictors of pain and interference in other chronic pain populations, for example, in musculoskeletal pain (Leeuw et al. 2007; Vlaeyen and Linton 2000), but have not been systematically analyzed in amputees with PLP.
12.5 The Role of Stress and Tension in PLP
It has been proposed that stress plays a role as a trigger of pain episodes in PLP and other types of chronic pain (Sherman et al. 1987; Flor and Turk 2011). However, the concepts of stress referred to in the literature are diverse and range from more broad constructs of general psychological distress (as used in Desmond and MacLachlan 2006), which bears similarities to anxiety and depression, to more situational and transient concepts like emotional and physiological arousal (as used in Angrilli and Köster 2000).
In a series of studies, Sherman (1994) and Sherman et al. (1989) examined the relationship of physiological alterations of the residual limb and PLP and proposed that local changes as well as autonomic system responses might contribute. For example, Sherman et al. (1987) showed that the temperature of the residual limb as compared to the intact limb is decreased in persons with PLP, most likely related to decreased near-surface blood flow. For burning, tingling, and throbbing pain, there was a significant relationship between the extent of the temperature difference and the intensity of PLP. Discharges of peripheral input can be mediated by autonomic nervous system activity, which could explain why situational components (like external stress) and internal states (like tension and anxiety) interact and trigger PLP episodes. Sherman et al. (1992) showed a close temporal relationship between (involuntary) contractions of the residual limb muscles and PLP. In this study, bursts, as recorded by surface electromyography signals, preceded the PLP experience. These rather involuntary contractions of residual limb muscles can be related to anxiety, tension, and stress.
Angrilli and Köster (2000) induced stress in amputees with and without PLP by having them report about memories of the amputation in a free speech and applied a cold pressor pain test and mental arithmetic as control tasks. Heart rate and blood pressure were recorded as a measure of sympathetic stress responses. Amputees with PLP showed a stronger psychophysiological stress reaction compared to amputees without PLP in the free-speech task. This study also supports the notion that distressing pain memories play a role in PLP (Katz and Melzack 1990; Flor 2002; Giummarra et al. 2011a).
An interesting question is how these findings on the relationship between physiological stress and PLP in the laboratory relate to a more naturalistic context and to more general (“everyday”) stressors. Giummarra et al. (2011b) had amputees complete a structured questionnaire on experienced triggers of PLP episodes. Most commonly reported (50 %) were behavioral triggers, like “forgetting” that the limb is amputated and trying to use the phantom. Thirty-seven percent reported triggers related to stimulation of the residual limb, such as movement, touch, or pressure. Emotional triggers such as emotional distress, exhaustion, or thinking of the missing limb were reported by 23 %. Additionally, 20 % reported influence by the weather, and 11 % reported referred sensations from the intact limb. These findings support the notion that PLP episodes follow emotional distress and that input from the residual limb is an important factor. However, these data are based on subjective reports and may follow preconceived notions rather than actual events. Rather than using a survey, Arena et al. (1990) conducted a longitudinal study employing pain and stress diaries acquired from 27 male amputees with PLP who completed them four times a day for 6 months. A cross-lagged correlational analysis was used to detect relationships between stress and PLP over time. In 74 % of the amputees, a significant relationship between stress and PLP was found. In 63 % of this sample, a simultaneous covariation of stress and pain was observed, in 44 % a change in pain preceded a change in stress, and in 37 % a change in stress preceded a change in pain. This study supports the interpretation that there is a bidirectional link between PLP and stress.
12.6 Cognitive Factors
Cognitive factors such as anticipation, expectations, beliefs, interpretations, appraisals, and coping strategies have been found to modulate chronic pain (Turk et al. 1983; Turk 1999; Flor and Turk 2011). In PLP little is known about the role of cognitive factors (Hill 1999), although catastrophizing, coping styles, memory processes, and body representation have been examined.
12.6.1 Body Representation in PLP
The amputation of a limb is accompanied by alterations in body representation such as a changed perception of the missing limb. Amputees in general (Nico et al. 2004) and especially those experiencing PLP (Reinersmann et al. 2010) show an increase in reaction time when asked to mentally rotate a hand representing their missing limb. This delayed response has been viewed as indicative of a dysfunction in the processing of the body schema, which refers to more implicit aspects of body representation compared to the body image, which has been viewed as an explicit, conscious percept. As implicit and explicit aspects of body representation are dynamic and closely interwoven and cannot always be separated, we prefer the term body representation. Other basic processes involved in body representation, however, seem to be less affected. Thus, amputees can be induced to perceive a rubber hand as belonging to their body, when the seen rubber hand is synchronously touched together with the hidden residual limb (Ehrsson et al. 2008). The underlying neuronal principles in amputees (Schmalzl et al. 2014) appear to be similar to those in healthy controls (Ehrsson et al. 2004). No significant differences between amputees with and without PLP related to the responsiveness to this so-called rubber hand illusion have been found (Ehrsson et al. 2008). The intact ability of amputees with PLP to perceive an artificial limb as belonging to their body might be useful for the treatment of PLP. For example, functional prosthesis use has been found to be negatively associated with both dysfunctional cortical reorganization and PLP intensity (Lotze et al. 1999; Weiss et al. 1999), indicating that restoring the amputee’s body integrity might alleviate or prevent PLP. There is evidence that the perception of ownership of the prosthetic device plays a mediating role in this process, with more intense ownership experience of the prosthesis being associated with lower levels of PLP (Kern et al. 2009). Similarly, sensory feedback from the prosthesis (Dietrich et al. 2012) might enhance perceived ownership. However, amputees who report a telescopic distortion of their phantom stated less often the occurrence of ownership sensations for the prosthesis (Giummarra et al. 2010), suggesting that the presence of a telescope might influence the effectiveness of PLP treatment as well. In line with this, Foell et al. (2014) reported that the presence of a telescope is an important predictor for the effectiveness of mirror therapy. During mirror therapy, patients perform movements with their intact limb in front of a mirror and have to mentally combine the seen movements with the self-executed movements of the phantom. Amputees who experienced a telescope failed to relate the seen movement to the felt movement and did not benefit from the mirror intervention (Foell et al. 2014). Since the sensation of a telescope is associated with similar reorganizational processes like PLP (Grüsser et al. 2001), this finding highlights the importance of body perception and its neural correlates for the treatment of PLP.
12.6.2 Memory for Pain
It has been proposed that “somatosensory memories” of non-painful and painful sensations in the missing limb play a role in phantom limb awareness and PLP (Katz and Melzack 1990; Katz 1992; Flor et al. 2006). Anderson-Barnes et al. (2009) proposed that “proprioceptive memories” could explain sensations in a phantom limb and that there might exist learned associations between proprioceptive memories and pain perceived before amputation. Before amputation, pain in the affected limb is common, for example, due to a tumor, vascular disease, or injury. Katz and Melzack (1990) suggested that these types of pain are encoded and can later be triggered, for example, by peripheral input from the residual limb, and experienced as PLP. Support for this comes from retrospective reports showing a relationship between reported memories referring to the phase before or during amputation and later phantom sensations (Katz and Melzack 1990; Giummarra 2011a). In Katz and Melzack’s (1990) study, almost 60 % of amputees who reported some kind of pain before the amputation also reported that painful sensations continued or recurred in the phantom limb. It has been proposed that neural plastic changes following long-term nociceptive input can be seen as a neuronal mechanism underlying pain memories (Flor 2003, 2008). Maladaptive plasticity associated with PLP might be more severe if chronic pain precedes the amputation. The concept of pain memories and pain prior to the amputation is closely linked to the question whether the formation of pain memories and subsequent PLP can be prevented if nociceptive input is blocked before amputation, for example, by the use of anesthetic drugs. However, the evidence on this is controversial (Ypsilantis and Tang 2010; Jensen and Nikolajsen 2000). In a prospective study, Jensen et al. (1985) and Nikolajsen et al. (1997) showed that PLP during the first 6 months but not long-term PLP was predicted by pain before amputation. However, usually long-standing pain was not taken into account.
12.6.3 Catastrophizing
Pain catastrophizing is an exaggerated, negative orientation towards pain and has been found to predict chronic pain and impairment (Flor et al. 1993; Sullivan et al. 1995; Linton and Shaw 2011) as well as a negative outcome (Linton and Shaw 2011; Wertli et al. 2014). In several studies, catastrophizing has been shown to be significantly positively correlated to the magnitude of PLP in amputees (Hill 1993; Hill et al. 1995; Jensen et al. 2002; Hanley et al. 2004; Richardson et al. 2007; Vase et al. 2011, 2012). Jensen et al. (2002) and Hanley et al. (2004) showed that catastrophizing 1 month after amputation was correlated with concurrent PLP and depression. However, high catastrophizing was associated with an improvement of PLP and depression at 6 months or 2 years later. This finding seems contradictory. However, these results might be related to regression to the mean: due to the high correlation of PLP and depression with catastrophizing at the first time point, subjects with high values in catastrophizing show high values in depression and PLP as well, leaving subjects little chance to further aggravate. Hence, the lagged relationships in these two studies should not be interpreted in a way that catastrophizing predicts improvement but rather that the initial magnitude of pain needs to be taken into account as well. Richardson et al. (2007) showed that catastrophizing before the amputation predicted PLP 6 months after the amputation such that a high degree of catastrophizing was associated with more PLP. Catastrophizing has also been examined with respect to coping with disability, showing that catastrophizing predicted physical and psychosocial disability in amputees (Whyte and Carroll 2004). Vase et al. (2011) showed that catastrophizing accounted for 35 % of the variance found in PLP even after statistically controlling for depression and anxiety. Moreover, catastrophizing also correlated with wind-up-like pain, elicited by pinpricks at the residual limb. The wind-up test is a dynamic pain measure in which moderately painful stimuli of the same intensity are repetitively presented at the same site. Usually, stimuli are perceived as increasingly painful. This measure is seen as an indicator of amplification of peripheral nociceptive input. The authors assumed that both catastrophizing and wind-up interact and contribute to PLP and that, given the trait-like nature of catastrophizing and the fact that it precedes PLP, catastrophizing might lead to wind-up. In another study (Vase et al. 2012), electroencephalography was used to record cortical responses to noxious and non-noxious stimuli presented at the affected and non-affected limb. For the affected side, there was a correlation between catastrophizing and the root mean square power of the N/P135 dipole, which was located in the area of secondary somatosensory cortex, known to play a role in attentional processes. The authors interpret this finding as an indicator that catastrophizing relates to hypervigilant attention for noxious and non-noxious stimuli.
12.6.4 Coping Strategies
Pain coping strategies describe various ways to “deal” with pain after it has been attended to and interpreted (appraised) as being a threat (Rosenstiel and Keefe 1983; Linton and Shaw 2011) and can be divided into cognitive and behavioral strategies. Examples of cognitive coping strategies are distracting attention from a sensation or reinterpreting pain (Hill 1993). Behavioral coping refers to strategies like increasing or decreasing social or physical activity or seeking social or medical support (Hill 1993; Linton and Shaw 2011). Coping with PLP was first systematically been studied by Hill (1993) who used the Coping Strategies Questionnaire in 60 male amputees with PLP. A principal component analysis in the amputee sample revealed a factor structure that was similar to the one originally discovered for chronic back pain patients (Rosenstiel and Keefe 1983). Three main components were found which the authors called “cognitive coping,” “helplessness,” and “pain denial.” They explained about 20 % of the variance in both PLP and psychological distress. An analysis of subscales revealed that catastrophizing was by far the most powerful factor accounting for most of the variance explained by the “helplessness” factor. The authors concluded that PLP sufferers use a limited amount of coping strategies that help to alleviate distress and pain and that “successful” coping rather means not to catastrophize. In another study, Hill et al. (1995) found that catastrophizing explained 26 % of the variance in pain as opposed to other strategies that only explained 3 %. Whyte and Niven (2011b) examined 89 amputees with pain diaries assessing PLP and coping strategies. Unlike other studies, strategies were captured in a free format without standardized questions. Diary entries were made once per hour for 1 week. The participants used a limited number of strategies falling into the categories of distraction, relaxation, seeking support, exercise, manipulation of the residual limb, and drug or alcohol use. Interestingly, none of the reported strategies turned out to be effective in reducing PLP. This study confirms that PLP sufferers have few effective coping strategies.
12.7 Prediction and Prevention of PLP
Prospective studies have examined factors in the pre- or early post-amputation phase that might predict PLP. Parkes (1973) predicted PLP 13 months after amputation by a set of variables assessed in the first weeks after amputation. In addition to pain in the residual limb or phantom and health-related predictors such as having suffered from a life-threatening physical illness before amputation, “rigidity” and “compulsive self-reliance” were significant. However, other researchers have found no association of personality types and PLP (e.g., Sherman et al. 1987). Castillo et al. (2013) examined depression, anxiety, and pain in a sample of subjects with lower limb trauma at 3, 6, 12, and 24 months after injury. In this study, not all subjects were amputees, and the study did not differentiate between PLP and other types of pain. Pain predicted depression, but depression did not predict pain. However, anxiety predicted pain, especially in the later, chronic phase. The relationship between PLP, depression, and cognitive and social factors was studied by Jensen et al. (2002). Between 1 and 6 months after amputation, the change in PLP and depressive symptoms could be predicted by catastrophizing and lack of social support and overly solicitous responses from family members. In a later study (Hanley et al. 2004), these results were replicated for a period of 1 and 2 years following amputation. Hunter et al. (2008) examined skin temperature and tactile spatial acuity of the residual limb within the first 6 months and 1–3 years following amputation. There was no clear relationship between these measures and PLP or residual limb pain. The use of a functional prosthesis was associated with vivid phantom limb awareness, but not with decreases in PLP at follow-up.
Other studies predicted PLP by variables assessed before the amputation. Richardson et al. (2007) showed that catastrophizing predicted PLP 6 months after the amputation, whereas pain before the amputation was only weakly related. Prediction of PLP by means of pain before amputation was also investigated by other researchers (Jensen et al. 1985; Nikolajsen et al. 1997) who found a relation only 6 months, but not later after the amputation. Similarly, the sensitivity to pressure pain stimuli at the residual limb before an amputation predicted PLP at 1 week, but not at 6 months after amputation (Nikolajsen et al. 2000).
12.8 Plastic Changes and the Role of Emotional and Cognitive Factors
So far, an integration of physiological and psychological factors in PLP is lacking although it can be assumed that these factors interact. We have previously summarized important peripheral and central factors involved in PLP (see Fig. 12.2). We suggest that they influence both the representation of pain in the central nervous system and specifically in cortical areas and also the peripheral input that contributes to these changes, for example, through sympathetic activation. Flor and Turk (2011) and Simons et al. (2014) have summarized additional factors such as motivation or learning and conditioning processes that are important in the understanding of chronic pain, but these have not yet been examined in PLP. For example, Diesch and Flor (2007) showed that Pavlovian fear conditioning alters the map in primary somatosensory cortex such that the conditioned stimulus that predicts pain occupies a larger area and shifts the activation map towards the representation of the unconditioned stimulus.
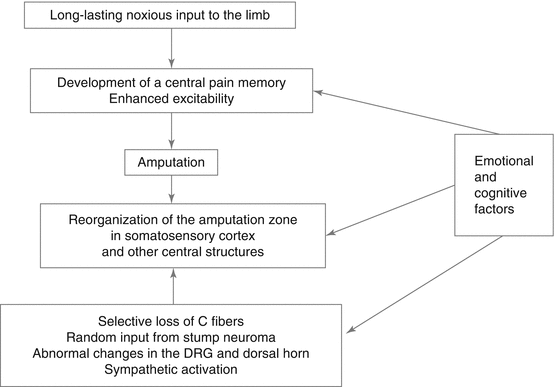

Fig. 12.2




Central and peripheral factors assumed to contribute to phantom limb pain and their interaction with emotional and cognitive factors
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





