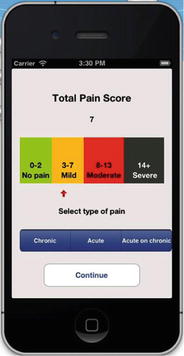
Fig. 7.1
A classic comment in the literature
Many healthcare professionals and patients themselves tend to assume that pain is a natural part of the ageing process and so there is no need to do anything about it (Crook et al. 1984; Herr and Mobily 1997; Hofland 1992; Morrison and Siu 2000). Add into this mixture the fact that the individual may not communicate their pain in a language that is easily understood by healthcare professionals.
It is worthwhile to remember that pain is a complex phenomenon and can be influenced by psychosocial and cultural factors, so individuals may choose not to report their pain. But asking the question in various formats may provide an opportunity for those in pain to be able to report their pain. It may be appropriate to ask the question in different ways (Feldt et al. 1998). For example, (Fig. 7.2).
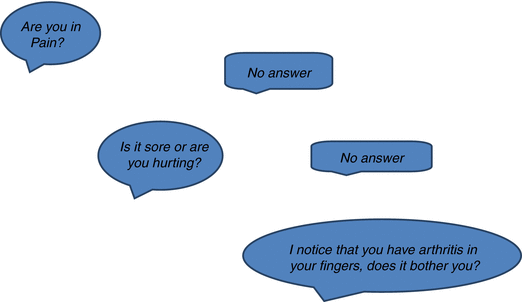

Fig. 7.2
Asking about pain
7.3 Pain Evaluation: Types of Scales for Assessing Pain
1.
Self-report – Numerical rating scale (0–10) or verbal rating scale (none, mild, moderate, severe) has high validity and reliability in older people (Herr and Mobily 1993; Herr et al. 2007). They can be used in mild/moderate cognitive impairment (Weiner et al. 1999). Vertical as opposed to horizontal orientation may help to avoid misinterpretation in the presence of visuospatial neglect, e.g. in patients with stroke.
2.
Older people with moderate to severe cognitive impairment – Pain thermometer or coloured visual analogue scale is easy to use in those with cognitive/communication impairment (Herr et al. 2007). Validity has not been fully evaluated but is well understood in early- and mid-stage Alzheimer’s disease.
3.
4.
Multidimensional assessment – Older people with minimal cognitive impairment: Brief Pain Inventory is a 15-item scale assessing severity, impact on daily living, impact on mood, and enjoyment of life (Auret et al. 2008).
As discussed earlier, it is anticipated that there will be a significant proportion of the older population experiencing dementia in the future, and this will impact upon our ability to identify and subsequently manage pain. Often behaviour in adults with dementia is perceived as “challenging”, and historically, such behaviour has been treated with antipsychotic medications. Certainly, in the UK, there has been a move away from prescribing such drugs, and healthcare professionals are becoming more aware of changes in behaviour associated with other problems such as pain or distress. In fact recently, the work by Hyochol and Horgas (2013) proposed that “challenging” behaviour can be reduced when treated with analgesic drugs. This study evaluated the minimum data set measures taken in all nursing homes within the USA and demonstrated that residents with more severe pain are less likely to display wandering behaviours, but more likely to display aggressive and agitated behaviours. The authors concluded that the “relationship between pain and disruptive behaviours depends on the type of behaviours”. Pain is positively correlated with disruptive behaviours that do not involve locomotion (e.g. aggression and agitation), but negatively related to disruptive behaviours that are accompanied by locomotion (e.g. wandering) (Hyochol and Horgas 2013). Some of the common behavioural signs reported in the literature include the following: Verbal communication – shouting, screaming, crying; Nonlanguage-based verbal – grunts, groans, aggression, agitation, withdrawal, facial expression, protecting an area; Physiological signs – pallor, sweating, blood pressure, pulse (not always present).
Such signs do not necessarily require a “trained eye”; they are signs that could be observed by any person. Nevertheless, they are signs that have often been incorporated into behavioural pain assessment tools. These will be discussed further below.
A recent systematic review of the literature was carried out in 2007 for the UK National Pain Assessment Guidelines (Collett et al. 2007). Within this guidance, two self-report pain intensity scales were recommended as appropriate for the measurement of pain in older adults with mild to moderate dementia. The scales were verbal descriptors (none, mild, moderate, severe) and numerical rating scale 0–10 scale.
It may be necessary to use both scales, if one does not work. But these are the two scales with the most evidence underpinning their use. Furthermore, a recent systematic review in 2013 was carried out to update the UK National Pain Assessment Guidance, and these scales continue to be the recommended with the strongest evidence (Schofield et al. 2014).
If severe dementia is present or language skills are lost, then it may be necessary to use one of the behavioural scales. In total, there are around 12 scales within the literature and all are fairly similar in terms of the behaviours that they attribute to pain. In 2007, the scale that had the most evidence was the Abbey scale.
7.4 Use of the Abbey Pain Scale
The Abbey pain scale is best used as part of an overall pain management plan (Collett et al. 2007). The Pain scale is an instrument designed to assist in the assessment of pain in residents who are unable to clearly articulate their needs. The scale does not differentiate between distress and pain, so measuring the effectiveness of pain-relieving interventions is essential. Recent work by the Australian Pain Society recommends that the Abbey pain scale be used as a movement-based assessment (Australian Pain Society 2005). The staff recording the scale should therefore observe the resident while they are being moved, e.g. during pressure area care, while showering, etc. The scale should be completed immediately following the procedure and record the results in the resident’s notes. Include the time of completion of the scale, the score, staff member’s signature, and action (if any) taken in response to results of the assessment, e.g. pain medication or other therapies. A second evaluation should be conducted 1 h after any intervention is taken in response to the first assessment, to determine the effectiveness of any pain-relieving intervention. If, at this assessment, the score on the pain scale is the same, or worse, consider further intervention and act as appropriate. Complete the pain scale hourly, until the resident appears comfortable, then 4 hourly for 24 h, treating pain if it recurs. Record all the pain-relieving interventions undertaken. If pain/distress persists, undertake a comprehensive assessment of all facets of resident’s care and monitor closely over a 24-h period, including any further interventions undertaken. If there is no improvement during that time, notify the medical practitioner of the pain scores and the action/s taken.
An update of the UK National Pain Assessment Guidelines has revealed that there has been some more recent work using the Abbey scale which shows use of the observer scales such as Abbey, PAINAD, and NOPAIN improves assessment of pain in older adults with cognitive impairment (Lukas et al. 2013a). Furthermore, in the UK, Abbey scale is most widely accepted in practice. However, the recent systematic review due to be published in 2014 has identified that other scales have now overtaken the Abbey scale and perhaps the most credentialed and recommended behavioural pain assessment tool is now the PAINAD (Schofield et al. 2014).
As discussed earlier, in 2007 we identified 12 behavioural pain scales (Abbey, PAINAD, PACSLAC, DisDAT, PADE, PAINE, Doloplus, NoPain, CNPI, ADD, Mobid, and COOP). The recent update of the UK guidelines has identified 15 scales (Schofield et al. 2014). A further few scales have since been developed. In 2007, the Abbey, PAINAD, and Doloplus scales were recommended based upon the best evidence at the time. More work has been carried out using PACSLAC (Cheung and Choi 2008; Schiepers et al. 2010; Zwakhalen et al. 2012; Lints-Martindale et al. 2011) and PAINAD (Horgas and Miller 2008; Jordan et al. 2009; Lane et al. 2003; DeWaters et al. 2008). The PACSLAC scale has good inter-rater reliability (Cheung and Choi 2008) and is the scale most valued by nurses (Zwakhalen et al. 2012) but does need a short form and more testing in larger-scale studies. PAINAD is a sensitive tool for detecting pain in adults with dementia but does have a high false-positive rate (Jordan et al. 2009). The PAINAD has a high sensitivity (92 %) but low specificity for pain (62 %). It is easy and simple to use, although further research with larger sample sizes is required. The Doloplus scale has been translated in five languages (Pickering et al. 2010) and the Doloplus team recently developed the Algoplus scale. This scale has been developed specifically for acute pain measurement in adults with dementia (Rat et al. 2011). It was developed using expert opinion, caregiver interviews, and video recordings of patients. It is currently being validated in 5 languages and translated in 20 languages. The advantage of this scale is the very brief rater time (1 min). Therefore, it certainly shows a great deal of potential for clinical and research settings (Rat et al. 2011). It has become so popular in France that it has now overtaken the use of the Doloplus scale. Therefore, the Doloplus team is currently finishing a concordance study between both scales and data will be available soon. Moreover work by Schofield and colleagues within the COST collaborative on pain and dementia demonstrates that there is great inconsistency across European care homes in terms of the types of pain assessment scales being used and whether any pain assessment is undertaken at all, largely attributed to lack of education of aged care staff. The COST Action on pain and dementia will make future recommendations regarding pain assessment and implementation of assessment scales (http://www.cost.eu/COST_Actions/isch/Actions/TD1005 accessed 11th Nov 2014).
7.5 The Pain APP
Following the UK National Pain Assessment Guidelines, it was found that pain was not being widely assessed in adults with cognitive impairment across all care settings. Therefore, the pain assessment application was developed for Android or iPhone use. This follows the pain algorithm developed for the national guidelines in 2007.
7.6 Main Guidelines
In summary, pain assessment and management continues to be suboptimal across many countries (Lukas et al. 2013a, b; Schofield et al. 2014). This is in spite of a proliferation of pain assessment tools, many of which have been developed for older adults with communication difficulties including dementia. It is widely recognised that there is huge shift anticipated in the age cohort across the world with increased numbers of older adults compared to diminishing numbers of younger adults. Furthermore, it is anticipated that there will be a significant increase in the numbers of older adults with dementia over the next 20 years (Prince 2013) which further complicates the pain assessment process. A number of factors need to be considered when assessing pain in older adults with or without cognitive impairment as follows:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree