Peripheral Nerve Blockade
Ban C.H. Tsui
Richard W. Rosenquist
Key Points
Related Matter
Neck Anatomy
Brachial Plexus Block
Interscalene Block
Supraclavicular Block
Sensory Innervation of the Hand
TAP Block
Penile Block
Femoral Nerve Block
Ultrasound-Guided Saphenous Nerve Block
Ultrasound-Guided Popliteal Nerve Block
Ankle Block
Introduction
Advancements in medical knowledge and techniques are constantly being made and, while new advancements provide an opportunity for improved patient care, they need to be studied and compared to currently accepted techniques to evaluate their safety and utility. In contrast, anatomic structures are static, and an understanding of basic anatomy cannot be replaced by excellent technical skills and knowledge of the technique when performing regional anesthesia. Thus, this chapter provides an in-depth discussion of regional anatomy, while providing an overview of today’s two most up-to-date techniques for nerve localization and block performance: Nerve stimulation (NS) and ultrasound (US) imaging. Specific techniques that are practically useful for the anesthesiologist are described in sections grouped by body location.
General Principles and Equipment
Patient monitoring and other factors related to optimizing patient care and prevention of complications are similar to those for general anesthesia, with some important differences. Safe and successful performance of PNB requires careful selection of patients and administration of an appropriate type and dose of local anesthetic in the correct location. In addition, the patient must be monitored during the procedure and prior to discharge, and ambulatory patients with home going catheters should be monitored remotely with either telephone follow-up or home health care team visits until the catheter has been removed and the block has completely regressed.
Setup and Monitoring
Setup
Although regional blocks can be performed in the operating room setting just like general anesthesia, it is preferable and desirable to perform these techniques in a designated room or area outside the immediate operating room environment (Fig. 35-1). This is a consequence of what is commonly referred to as “soak time,” which is the time it takes for local anesthetics to cross the cell membrane, block action potentials, and produce either analgesia or surgical anesthesia. The designated area must contain the necessary equipment for safe monitoring and resuscitation but must also contain all of the supplies and equipment to perform common and sophisticated regional block techniques. Some important considerations for this “block room” are described below:
All supplies located in this area must be readily identifiable and accessible to the anesthesiologist.
The area should be of ample size to allow block performance, monitoring, and resuscitation of patients.
There should be equipment for oxygen delivery, emergency airway management and suction, and the area should have sufficient lighting.
A practically organized equipment storage cart (Fig. 35-1) is desirable and should contain all of the necessary equipment (including that required for emergency procedures), supplies, local anesthetics, needles, nerve stimulators, block trays, dressings, and resuscitation drugs. A US machine should also be present.
It is ideal to have a prepared specialty tray that includes items for sterile skin preparation and draping, a marking pen and ruler for landmark identification, needles and syringes for skin infiltration, and specific block needles and catheters.
A selection of sedatives, hypnotics, and intravenous anesthetics should be immediately available to prepare patients for regional anesthesia. These drugs should be titrated to maximize benefits and minimize adverse effects (high therapeutic index); short-acting drugs with a high safety margin are desirable.
Emergency drugs should include atropine, epinephrine, phenylephrine, ephedrine, propofol, midazolam, succinylcholine, and intralipid. In addition, guidelines for resuscitation in the setting of local anesthetic toxicity should be laminated and kept with the intralipid.
Monitoring
When performing regional anesthesia, it is vital to have skilled personnel monitor the patient at all times. At minimum, standard monitoring should include electrocardiogram (ECG), noninvasive blood pressure (NIBP), and pulse oximetry. In addition, the patient’s level of consciousness should be gauged frequently using verbal contact since vasovagal episodes are common during many regional procedures. At present, there are no practical or effective devices that detect rising blood levels of local anesthetic; however, this can be done indirectly by adding pharmacologic markers, such as epinephrine, in appropriate concentrations to the local anesthetics. Close observation for systemic toxicity secondary to rapid intravenous injection (within 2 minutes) as well as delayed (∼20 minutes) absorption is essential. The patient should be monitored for at least 30 minutes following the procedure.
Standard ECG and pulse oximetry are essential monitors when performing regional anesthesia.
Careful monitoring of the patient’s heart rate (along with ECG measurement) is important to detect tachycardia seen with epinephrine when it is included in a test dose. It is also useful as an indicator of systemic toxicity with bupivacaine and other potent local anesthetics.
Before performing blocks with significant sympathetic effects, a baseline blood pressure reading should be obtained. Once the regional anesthesia procedure is complete, the monitors should remain attached. In conscious patients, end-tidal carbon dioxide monitoring is not required; however, there are special nasal prongs available for monitoring patients when this is considered necessary.
At minimum, stable vital signs must be present following regional anesthesia to fulfill discharge criteria from the recovery area. If the block has not begun to regress, appropriate protection for the anesthetized limb and complete instructions should be provided to the patient and their family before discharge. For inpatients, appropriate orders should be written to assure limb protection.
Patients receiving perineural local anesthetic infusions should be visited regularly by a qualified physician postoperatively (i.e., Acute Pain Service) with ongoing documentation of their condition in the medical record.
Common Techniques: Nerve Stimulation and Ultrasound Imaging
Nerve Stimulation
Basics of Technique and Equipment
Electrical stimulation of nerve structures was introduced to regional anesthesia in the middle of the 20th century.9,10 A low-current electrical impulse applied to a peripheral nerve produces stimulation of motor fibers and theoretically identifies proximity to the nerve without actual needle contact or related patient discomfort. When NS techniques are used, it is unnecessary to make actual contact with the nerve (in contrast to the paresthesia method). This theoretically infers that the risk of nerve injury should be less when using NS methods, although this theory has not been proven. Stimulating catheters have recently been introduced and have increased our ability to accurately advance catheters over greater distances along nerve structures.11,12
Today’s nerve stimulators have features to improve ease-of-use and success, such as maintaining a constant current with adjustable frequency, pulse width, and current intensity (milliamperes; mA). This enables a stable current output (an important safety feature) in the presence of varied resistances from the needle, tissues, and connectors. A clear digital display indicating the actual current delivery is important, as is regular calibration and testing. Some nerve stimulators are equipped with low (up to 6 mA) and high (up to 80 mA) current output ranges. The lower range is primarily for localizing peripheral nerves, while the higher range is mainly used for monitoring neuromuscular blockade. Recently, higher ranges have been utilized for transcutaneous NS techniques17 (2 to 5 mA) including percutaneous electrode guidance18 and surface nerve mapping,11,19 and the epidural stimulation test (1 to 10 mA).20,21 Most nerve stimulators deliver an electrical pulse width of 100 or 200 μs for stimulating motor nerves. Similar to current amplitude, the length of time over which the current is delivered (pulse width) is usually considered important, as shorter duration currents can selectively stimulate motor components of mixed nerves while sparing the discomfort caused by stimulation of sensory components. Some sophisticated devices allow variable pulse widths from 50 μs to 1 ms in an attempt to provide such selective stimulation. The general rule is to use short-duration current of ≤100 μs for peripheral NS, although there is some evidence that duration does not impact patient discomfort22 and that intensity (mA) of the stimulation is perhaps the most important variable.23
Practical Guidelines
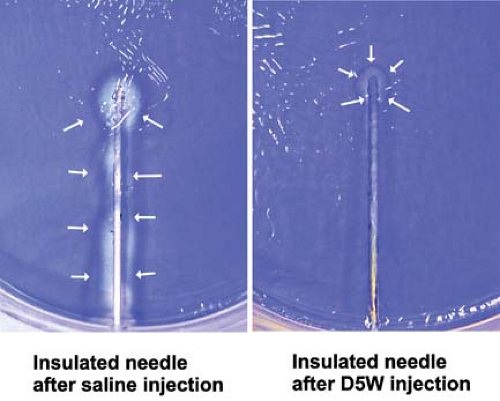
During initial advancement of the needle, the nerve stimulator should be set to deliver a current of 1 to 2 mA in order to gauge the approximate distance to the nerve. Depolarization of the nerve can also be improved by using the positive (anode; red) pole of the stimulator as the ground (reference or surface electrode) electrode and the negative (cathode; black) lead as the connection to the needle itself (known as cathodal preference). The actual location of the ground is of little importance with the use of constant-current nerve stimulators.23 Generally, the needle is in close proximity to the nerve when the threshold for motor response is between 0.3 and 0.5 mA; placing the needle to the point where a motor response only requires 0.1 to 0.2 mA may increase the chance of intraneural injection and should be avoided.24 Once a low threshold response is obtained, 2 to 3 mL of local anesthetic is injected and the operator watches for disappearance of the motor twitch, which is a signal to inject the remainder of the proposed dose in divided aliquots. This “Raj test”25 was originally thought to result from physical displacement of the targeted nerve by the injectate, but this response has recently also been attributed to a change in the electrical field at the needle-tissue interface. Electrically conducting solutions (e.g., local anesthetic or saline) reduce the current density at the needle tip, thereby increasing the current threshold for motor response, whereas nonconducting solutions (e.g., dextrose 5% in water; D5W) increase the current density and maintain or augment the twitch response (Fig. 35-2).26
After nerve localization using a stimulating needle, introduction of a stimulating catheter with continuous stimulation of the nerve is suitable to provide continuous analgesia. Similar current thresholds are applicable with the use of stimulating catheters. If an attempt to dilate the perineural space is undertaken, injection of D5W is preferable in order to maintain the motor response to stimulation.27 The reader is referred to the section on Other Related Equipment for optimal features of stimulating catheters.
Ultrasound Imaging
Basics of Technique and Equipment
US imaging is rapidly emerging as a very promising regional anesthesia tool since the size, depth and precise location of many nerves in their surrounding environment can be determined with correct interpretation of the visual image. Visualization of the moving needle, once inserted at an appropriate angle and within the plane of the US probe, as well as the spread of local anesthetic, provides valuable assistance to the anesthesiologist performing regional anesthesia. With US-guided PNB techniques, the operator can adjust needle or catheter placement under direct vision (i.e., US imaging), which may lead to fewer needle attempts and ultimately improved motor and sensory blocks. Furthermore, visibility of vital structures (e.g., blood vessels and pleura) is advantageous in order to avoid complications. Today, technologic advances have led to the development of US systems that can deliver high frequency (10 MHz or higher) sound waves offering the high axial resolution required for visualization of nerves and the ability to distinguish them from the surrounding anatomical structures (e.g., tendons, muscles). The proposed benefits of US guidance, as compared to NS, for upper extremity blocks include improved block success28 and completeness,29 reduced block performance and onset times,28,29,30,31 prolonged duration of blocks,30 and reduction in complications.32 While the cumulative evidence may appear convincing, many of the studies show conflicting results for certain parameters, and the large variability in trial methodologies and application of different outcome measures account for many of the discrepancies. Indeed, the various endpoints used during research in regional anesthesia may bias outcomes when comparing multiple regional techniques. Recently, Marhofer et al.33,34 published an excellent review of the current status of US and its use in regional anesthesia. They emphasize that adequate training in US-guided techniques is essential and suggest that education and proper technique can help ensure safe blocks. In addition, current advantages of using US in regional anesthesia, including direct visualization of subcutaneous structures, identification of anatomic variation, ability to use less local anesthetic, and improvement in block quality and patient satisfaction, are also discussed.
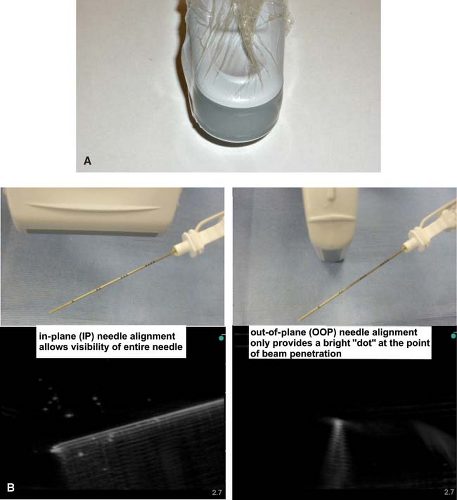 Figure 35-3. A: Probe sterility using a sterile transparent dressing (e.g., Tegaderm; 3M Health Care, St Paul, MN) without the full cover of a sterile sleeve. Other dressings may create multiple small wells over the probe surface due to adhesive pockets and lead to poor image quality.36 B: In-plane and out-of-plane needle alignment and subsequent visibility of the needle. |
US is defined as any sound with a frequency >20 kHz, although medical imaging generally requires between 3 and 15 MHz. Within the body, US scanners emit sound waves that produce an echo when they encounter a tissue interface. Therefore, US images reflect contours, including those of anatomic structures, based on differing acoustic impedances of tissue or fluids. Significant reflection of sound waves occurs at interfaces between substances of different acoustic impedance, resulting in good contour definition between different tissues. High US beam reflection, from high impedance/dense structures (e.g., bone, connective tissue), results in a bright (hyperechoic) image, often with dorsal shadowing underneath; low impedance structures reflect beams to a smaller extent and appear gray (hypoechoic); minimal impedance structures/spaces (e.g., fluid in vessels) appear black (anechoic).
Higher frequencies offer the best spatial resolution at superficial locations (e.g., brachial plexus at supraclavicular fossa), while lower frequencies are often required for structure delineation at deep locations (e.g., sciatic nerve in the subgluteal region). Block location and depth of target nerve structures determine which transducer offers the best imaging and resolution. Several functions of the US system are important to have familiarity with, including field and gain functions as well as Doppler effect. Doppler effect is very useful for identifying blood vessels during nerve localization using US guidance, since many nerves are situated in close proximity to vascular structures.
Practical Guidelines
Both the probe and the skin of the patient should be prepared for maximum sterility and optimal imaging. Probe sterility is paramount if performing real-time, or dynamic, US guidance during block performance. This can be maintained by standard sleeve covers but these can be expensive and cumbersome. For single-shot blocks, it is practical to use a sterile transparent dressing (e.g., Tegaderm; 3M Health Care, St Paul, MN) without the full cover of a sterile sleeve (Fig. 35-3A).35 An issue when using standard long covers is the potential for air to track between the probe and the skin, which reduces image quality. The target area should be surveyed (scanned) using a generous amount of US gel (water soluble conductivity gel is optimal) prior to sterile preparation. One of the most common reasons for poor visualization is lack of sufficient gel for skin-probe contact.
proceed to follow along, or “trace” the nerve to the optimal block location (Table 35-1).36,37 Generally, nerve structures are most visible when the angle of incidence is approximately 90 degrees to the US beam. Obtaining a transverse axis view of the nerve usually allows the best appreciation of the anatomical relationship of the nerve with its surrounding structures. To obtain the best possible view of the shaft and tip of the needle, it is imperative to align the needle shaft to the longitudinal axis (“in-plane”; IP) of the US transducer (probe) (Fig. 35-3B). The nerve structure is often placed at the edge of the US screen to ensure adequate viewing distance for the needle shaft. An alternative approach uses a transverse or tangential (“out-of-plane”; OOP) alignment, which only allows appreciation of the needle in cross section and usually only during movement (Fig. 35-3B). The nerve structure is often placed in the center of the screen to guarantee that aligning the needle puncture with the center of the probe will ensure close needle tip-nerve alignment. This approach can be beneficial in certain block locations (compact areas) and for inserting catheters (e.g., at the subgluteal area), but should never be used in areas where needle tip visibility in relation to vital structures is critical (e.g., supraclavicular fossa near the pleura).
Table 35-1. Useful Anatomical Landmarks for Localizing Nerves During Common Ultrasound-Guided Peripheral Nerve Blocks | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
After the needle is seen to be close to the nerve(s), a 1- to 2-mL test dose of local anesthetic or D5W can be injected to visualize the spread and perform a “Raj test” if a stimulating needle is being used. The solution will be seen as a hypoechoic expansion and will often illuminate the surrounding area, enabling better visibility of the nerves and block needle. If NS is being used to confirm nerve identity, it is useful to administer D5W in order to maintain accurate motor responses.27 This will be especially important during catheter introduction and advancement. If the test shows undesired injection near or within vessels or cavities, subsequent injection of local anesthetic should be postponed until better needle localization is achieved. If suboptimal spread of injectate is observed, the needle can be repositioned to allow another injection.
There can be a lengthy learning curve for US-guided nerve blocks, and techniques to improve needle and catheter visibility during
advancement are important in order to improve training for this technology. Two such approaches have been described experimentally:
advancement are important in order to improve training for this technology. Two such approaches have been described experimentally:
The “walk-down” approach to facilitate needle tip identification during OOP needling.37,38 This technique involves calculating the required depth of puncture (with measurement to the desired neural structure recorded using US prior to the block) and using trigonometry with the shaft angle and length to calculate a “reasonable” location to place the initial needle puncture site. The initial shallow puncture will be easily seen as a bright dot on the screen, and the needle tip can be followed as it is “walked down” to the final calculated depth. For example, if the final depth of penetration for the block is 2 cm, the needle will ultimately obtain a 45-degree angle if the initial puncture site is 2 cm from the probe and the needle is incrementally angled to this level.
A method of needle-probe alignment using a laser attachment for the probe has been reported; the laser line will project onto both the needle shaft and the midline of the probe, indicating an IP position.37,39 Aligning the visible optical laser line with the longitudinal axis of the US probe will mimic the “invisible” beam from the US probe and allow improvements with IP needle alignment. With the laser-unit attachment, any misalignment of the needle to the US beam can be easily detected and adjusted in real-time. Recently, commercially available GPS guidance systems intended to guide the needle tip location have been developed, but the merit of these devices remains to be determined.
Other Related Equipment
Needles
Needles used for regional techniques are often modified from standard injection needles. Although reports may speculate that needle design is a determinant of nerve or other tissue injury, there is insufficient evidence to fully substantiate this claim. For PNB, the “short bevel” (i.e., 30 to 45 degrees) or “B bevel” is often used to reduce the potential for injury to nerves.40 Other modifications, such as the “pencil-point” needle, have been introduced in attempts to reduce nerve injury. Single-shot PNB techniques generally require using 22- to 24-gauge insulated needles with short bevels. If superficial and field blocks are performed, smaller gauge (e.g., 25 to 26 gauge) sharp needles can be utilized. Continuous blocks require larger-bore needles to facilitate catheter introduction (e.g., 18-gauge needles for 20-gauge catheters). Blunt-tipped Tuohy needles are commonly used for continuous PNB with success.41 Short-bevel and Tuohy needles offer more resistance and give a better “feel” when traversing different tissues. Desired needle length will depend on each specific block and individual patient characteristics. Clear markings throughout the entire length of the needle are important for measuring depth of penetration, particularly for correspondence to US measurements.
Practical Tips
Techniques and devices have been proposed to limit injection pressure, since there is considerable variation among anesthesiologists in the amount of pressure they apply during injections42 and high-pressure injections into the nerve (especially intrafascicular) have been associated with damage in animals.43,44 Disposable, in-line injection pressure monitors are available, although their ability to prevent long-term injury is not well documented. Alternatively, a compressed air injection technique (CAIT) has been described to limit the generation of excessive pressure during injection. With this method, air is drawn into the syringe and compressed by 50% during the entire injection to maintain pressures of approximately 760 mm Hg (Boyle’s law: Pressure × volume = constant).45
Catheters
Continuous-infusion catheter kits suitable for PNB are available that include a standard polyamide catheter, such as those previously used for epidural analgesia, combined with an insulated Tuohy needle with NS capability. Recently, catheters have been advanced to the point of making them amenable to stimulation (an electrode is placed into the catheter tip). This may enable more accurate advancement of catheters for substantial distances to provide continuous analgesia. Some studies have suggested that it may be helpful to inject a solution to dilate the perineural compartment to facilitate the advancement of catheter. The reader is referred to the discussion of practical guidelines of NS in the section Common Techniques: Nerve Stimulation and Ultrasound Imaging for discussion of injection solutions for perineural dilation. There are a number of continuous-infusion devices now available for both inpatient and outpatient use, which allow delivery of dilute local anesthetic concentrations for as long as 72 hours after surgery. Standard precautions are required to maintain sterility of the catheter and the insertion site, but complications have been rare with these techniques and new devices.
Avoiding Complications
In general, regional anesthesia has an excellent safety record. Complication rates are as low as 8 per 10,000 for seizures2 and <0.1% to 1% for nerve injury,7,46 and only rare cases of severe chronic pain syndromes following regional anesthesia have been reported.47 Nevertheless, the incidence of some complications is often higher in PNB than other regional anesthesia/analgesia techniques, and results can be devastating. Choosing a suitable patient and applying the right dose of local anesthetic in the correct location are the primary considerations. Careful attention to any unusual responses or report of pain during block performance as well as follow-up prior to and after discharge is equally important, although often overlooked.
Patient Selection
Patient selection is a critical element for the performance of safe and effective PNB. Not all patients are suitable candidates for PNB. In general, patients scheduled for extremity, thoracic, abdominal, or perineal surgery should be considered potential candidates for peripheral regional anesthetic techniques. Adamant refusal of regional anesthesia by a patient is a contraindication to the procedure.
Other contraindications include local infection, systemic anticoagulation, and severe systemic coagulopathy. In most cases, schizophrenic patients should only receive regional techniques if general anesthesia is also performed. The presence of pre-existing neurologic disease is a controversial topic and, while a limited amount of data is available in the case of spinal anesthesia, the safety of PNB is unclear. One must be cognizant of the potential to compound existing neurologic deficit; therefore, clear documentation of the deficits prior to the procedure and a careful discussion of the potential risks and benefits are critical. For every clinical situation, the use of regional anesthesia must be carefully evaluated as a matter of risk versus benefit. It is imperative to follow applicable national and international guidelines, such as those set by the American Society of Anesthesiologists (ASA) for patient monitoring and those in place for anticoagulated patients, as provided by the American Society of Regional Anesthesia and Pain Medicine (ASRA).
Local Anesthetic Drug Selection, Toxicity, and Doses
This section will provide an overview of drug selection and toxicity during PNB. For a more detailed discussion of the
pharmacology and toxicity of local anesthetics, the reader is referred to Chapter 21.
pharmacology and toxicity of local anesthetics, the reader is referred to Chapter 21.
Systemic toxicity is most often related to accidental intravascular injection, and rarely to the administration of an excessive quantity of local anesthetic to an appropriate site. The risk of systemic toxic reactions is often related to the drug used. Ropivacaine (generally at 0.5%) is a recent example of a drug introduced into clinical practice in order to reduce central nervous system and cardiovascular toxicity through its physiochemical and stereoselective properties.50,51 Despite this, there are examples of ropivacaine toxicity during PNB.52,53,54,55 One strategy to potentially reduce the volume and concentration of local anesthetic solution required to produce a successful block is the use of US imaging to more accurately position the needle in close proximity to the nerve and to visualize the spread of solution to ensure adequate exposure.56,57 Of greatest importance is the ability to avoid intravascular injection. This risk may be reduced when using US, especially if combined with color Doppler for vessel localization.
The degree of systemic drug absorption and the duration of anesthesia can also vary depending on the site of injection (i.e., level of vascularization) and addition of vasoconstrictors. The highest blood levels of local anesthetic occur after intercostal blocks, followed by caudal, epidural, brachial plexus, intravenous regional, and lower extremity blocks. Equivalent doses of local anesthetic may produce only 3 to 4 hours of anesthesia when placed in the epidural space, but 12 to 14 hours in the arm, and 24 to 36 hours when injected along the sciatic nerve. Many believe that the addition of epinephrine (1:200,000 to 1:400,000) is advantageous in prolonging the duration of block and in reducing systemic blood levels of local anesthetic, although this has more relevance to local anesthetics like lidocaine and less to ones like bupivacaine. Its use is not appropriate in the vicinity of “terminal” blood vessels, such as in the digits, penis, or ear or when using an intravenous regional technique. Using significant quantities of local anesthetic during PNB should not be performed unless oxygen, suction, and appropriate resuscitation equipment is immediately available. However, even small doses of local anesthetic may produce significant side effects when injected into susceptible regions such as the neck. When performing PNB, a test dose of an epinephrine-containing solution and small incremental injections are recommended to reduce the risk of unrecognized intravascular injection. Toxicity can also occur from peripheral absorption of excessive doses of local anesthetic. Patients should be observed carefully for at least 30 minutes following injection because peak blood levels may occur at this time.
Animal studies58 and recent case reports59,60 have shown successful resuscitation from local anesthetic toxicity by intravenous administration of intralipid (20% lipid; not the 10% lipid of propofol), using one or more boluses (each of 1 to 2 mL/kg or 100 mL) followed by a 30-minute infusion (0.5 mL/kg/min). It is important to use this strategy as an acute resuscitation agent, only after standard measures have proven ineffective.
Nerve Damage and Other Complications
Peripheral nerve injury in humans may result from intraneural injection61,62 or direct needle trauma,63 although there are other causes, including those related to the surgical procedures (e.g., patient positioning, proximity of nerve to surgical site, and tourniquet application).64 Needle-related trauma without injection may result in injury of lesser magnitude than that from injection injury.65 In animal studies, nerve injury appears to occur when high injection pressures are applied intrafascicularly and particularly when highly concentrated local anesthetic solutions or their preservatives are used.43,44,66 One major sequela from intrafascicular injection is endoneural ischemia.67 While in some cases these syndromes resolve uneventfully, full recovery of some peripheral injuries may never occur or may require several months, a result of slow regeneration of injured peripheral nerves.61
Other minor complications that have been reported following PNB include pain at the site of injection and local hematoma formation, but these are self-limited side effects and are best dealt with by communication with the patient and reassurance by the anesthesiologist. A hematoma around a peripheral nerve is not of the same significance or risk as that occurring in the epidural or subarachnoid space. It is important to address concerns expressed by patients and to make every effort to relieve any pain or discomfort resulting from various interventions.
Discharge Criteria
Stable vital signs must be present in order to fulfill criteria for discharge from the recovery area. In some cases, acceptable evidence of regressing sensory and motor blocks should be present. However, if a long-lasting local anesthetic was used to perform the block or a continuous catheter with an infusion of local anesthetic is present, the block may not show evidence of regression at the time of discharge. Postoperative follow-up is important to confirm that neurologic function has returned to normal. If a deficit is suspected, early neurologic assessment is critical to determine the appropriate course of management.
Patients should have well-controlled pain upon discharge. Incorporating a standard level of pain relief (e.g., on a verbal rating scale) prior to discharge home or to the ward is prudent. Specific common risks for certain blocks should be discussed with the patient prior to discharge. When discharging patients from postanesthesia care units while an extremity is still anesthetized (e.g., the block was performed to provide extended analgesia), it is absolutely necessary to provide in-depth instruction related to the risks and their prevention (e.g., risk of burns to anesthetized areas will require avoidance of certain forms of cooking; potential for developing pressure neuropathies). A clear understanding of the information provided is important for both the patient and their caregivers. Written instructions including expected course, common side effects, and 24-hour contact information should be provided.
Premedication and Sedation
The best preparation for a regional technique is careful patient selection and ensuring that the patient is adequately educated and informed about the anesthetic and surgical procedures. Supplemental medication is often helpful. Appropriate sedation and analgesia is an essential part of successful regional anesthesia in
order to produce maximum benefit with minimal side effects. Effective sedation can be achieved with a variety of medications, including but not limited to propofol, midazolam, fentanyl, ketamine, remifentanil, alfentanil, or a combination of these drugs. The dosages should be titrated to reach an appropriate level of sedation for the individual patient, specific nerve block procedure and length of surgery. Some examples are listed below.
order to produce maximum benefit with minimal side effects. Effective sedation can be achieved with a variety of medications, including but not limited to propofol, midazolam, fentanyl, ketamine, remifentanil, alfentanil, or a combination of these drugs. The dosages should be titrated to reach an appropriate level of sedation for the individual patient, specific nerve block procedure and length of surgery. Some examples are listed below.
Bolus
Midazolam 1 to 2 mg (titrated up to 0.07 mg/kg)
Fentanyl 0.5 to 1 μg/kg
Alfentanil 7 to 10 μg/kg
Ketamine 0.1 to 0.5 mg/kg
In addition to the general comments about premedication discussed in earlier chapters, regional anesthesia techniques have special requirements. Sedation must be adjusted to the required level of patient cooperation. In the case of elicitation of a paresthesia (as during several blocks in the head and neck region) or electrical stimulation techniques, the level of sedation must be sufficient to allow the patient to identify and report nerve contact. Although a low dose of opioid (50 to 100 μg of fentanyl or equivalent) will help ease the discomfort of nerve localization, patient responsiveness must be maintained. This does not preclude the use of an amnestic agent; small doses of propofol or midazolam may provide excellent amnesia while maintaining levels of consciousness that still allow cooperation.
Clinical Anatomy
Head and Neck
Trigeminal Nerve
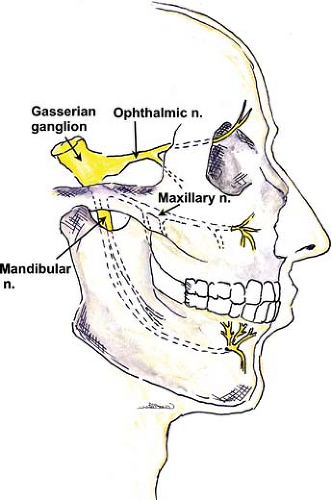
Sensory and motor innervation of the face is provided by the branches of the fifth cranial (trigeminal) nerve. The roots of this nerve arise from the base of the pons and send sensory branches to the large semilunar (trigeminal or Gasserian) ganglion, which lies on the dorsal surface of the petrous bone. Its anterior margin
gives rise to three main branches: The ophthalmic, maxillary, and mandibular nerves (Fig. 35-4). A smaller motor fiber nucleus lies behind the main trigeminal ganglion and sends motor branches to one terminal nerve, the mandibular. The three major branches of the trigeminal nerve each have a separate exit from the skull:
gives rise to three main branches: The ophthalmic, maxillary, and mandibular nerves (Fig. 35-4). A smaller motor fiber nucleus lies behind the main trigeminal ganglion and sends motor branches to one terminal nerve, the mandibular. The three major branches of the trigeminal nerve each have a separate exit from the skull:
The uppermost ophthalmic branch passes through the sphenoidal fissure into the orbit. The main terminal fibers of this sensory nerve, the frontal nerve, run to behind the center of the orbital cavity and bifurcate into the supratrochlear and supraorbital nerves. The supratrochlear branch traverses the orbit along the superior border and exits on the front of the face in the easily palpated supraorbital notch; the supraorbital nerve runs in a medial direction toward the trochlea (Fig. 35-5).
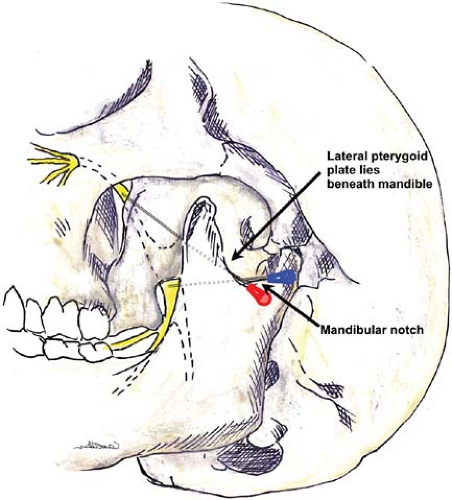
The maxillary nerve contains only sensory fibers. It exits the skull through the round foramen (foramen rotundum), passes beneath the skull anteriorly, and enters the sphenopalatine fossa. At this point, it lies medial to the lateral pterygoid plate on each side. At the anterior end of this channel, it again moves superiorly to re-enter the skull in the infraorbital canal in the floor of the orbit. It branches to form the zygomatic nerve, which extends to the orbit, the short sphenopalatine (pterygopalatine) nerves, and the posterior dental branches. The anterior dental nerves arise from the main trunk as it passes through the infraorbital canal. The terminal infraorbital nerve penetrates through the inferior orbital fissure to the base of the orbit, to the infraorbital groove and canal (just below the eye and lateral to the nose), and reaches the facial surface of the maxilla. It then divides into the palpebral (lower eyelid), nasal (wing of the nose), and labial nerves (upper lip).
The mandibular nerve is the third and largest branch of the trigeminal, and the only one to receive motor fibers. It exits the skull posterior to the maxillary nerve through the oval foramen (foramen ovale), forms a short thick trunk, and then divides into an anterior trunk, mainly motor, and a posterior trunk, which is mostly sensory. The main branch (posterior trunk) continues as the inferior alveolar nerve medial to the ramus of the mandible and innervates the molar and premolar teeth. This nerve curves anteriorly to follow the mandible and exits as a terminal branch (mental nerve) through the mental foramen. The mental nerve provides sensation to the lower lip and chin. Other terminal nerves include the lingual nerve (floor of mouth and anterior two-thirds of tongue) and the auriculotemporal nerve (ear and temple).
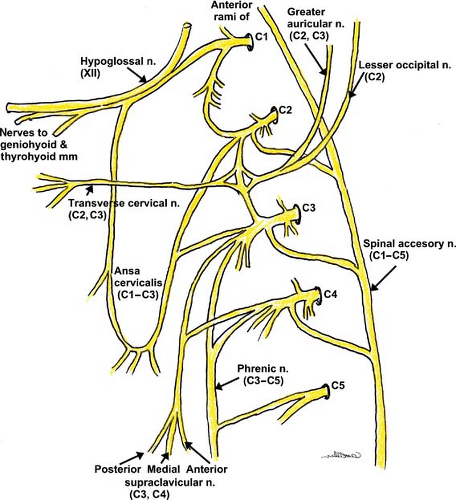
Cervical Plexus
Sensory and motor fibers of the neck and posterior scalp arise from the anterior rami (branches) of the first four cervical (C1–C4) spinal nerves. The cervical plexus is unique in that it divides early into cutaneous branches (penetrating the cervical fascia) (Fig. 35-6) and muscular branches (deeper branches that
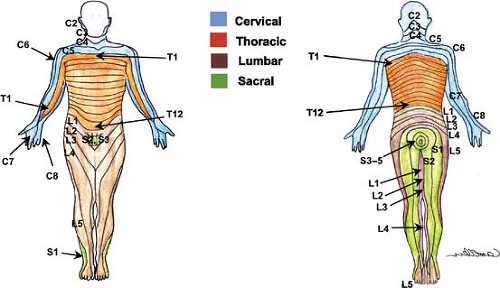
innervate the muscles and joints), which can be blocked separately (Fig. 35-7). The dermatomes of the cervical nerves C2–C4 are illustrated in Figure 35-8.
innervate the muscles and joints), which can be blocked separately (Fig. 35-7). The dermatomes of the cervical nerves C2–C4 are illustrated in Figure 35-8.
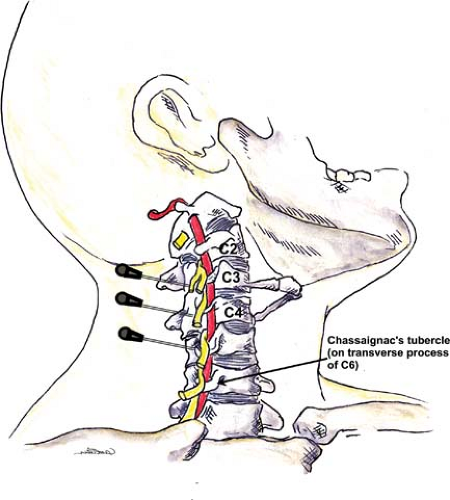
Classic cervical plexus anesthesia along the tubercles of the vertebral body produces both motor and sensory blockade. The transverse processes of the cervical vertebrae form peculiar elongated troughs for the emergence of their nerve roots. These troughs lie immediately lateral to a medial opening for the cephalad passage of the vertebral artery (Fig. 35-7). The trough at the terminal end of the transverse process divides into an anterior and a posterior tubercle, which can often be easily palpated.
These tubercles also serve as the attachments for the anterior and middle scalene muscles, which form a compartment for the cervical plexus as well as the brachial plexus immediately below. The compartment at this level is less developed than the one formed around the brachial plexus.
The deep muscular branches curl anteriorly around the lateral border of the anterior scalene and then proceed caudally and medially. Many branches serve the deep anterior neck muscles, but other branches include the inferior descending cervical nerve, the trapezius branch of the plexus, and the phrenic nerve, which give anterior branches to the sternocleidomastoid muscle as they pass behind it.
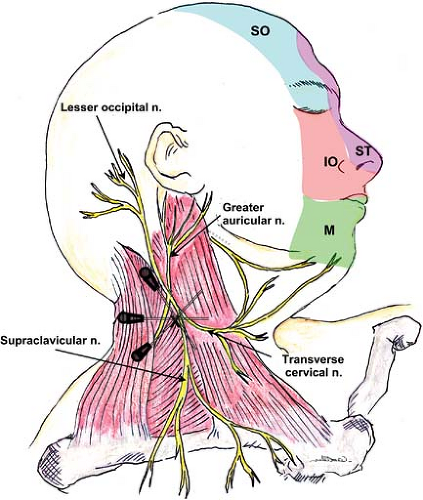
The sensory fibers emerge behind the anterior scalene muscle but separate from the motor branches and continue laterally to emerge superficially under the posterior border of the sternocleidomastoid muscle. The branches, including the lesser occipital nerve, great auricular nerve, transverse cervical nerve, and the supraclavicular nerves (anterior, medial, and posterior branches), innervate the anterior and posterior skin of the neck and shoulder.
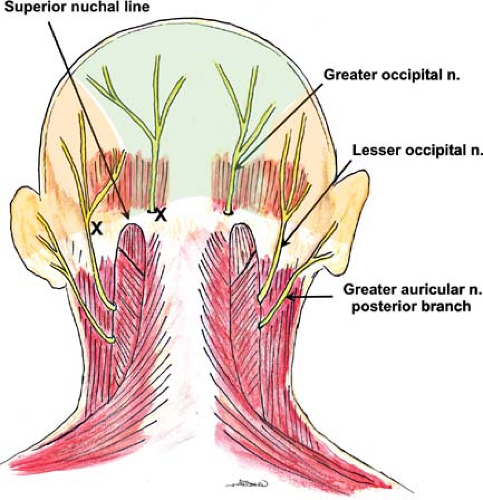
Occipital Nerve
The ophthalmic branch of the trigeminal nerve provides sensory innervation to the forehead and anterior scalp; the remainder of the scalp is innervated by fibers of the greater and lesser occipital nerves (Fig. 35-9).
The lesser occipital nerve arises from the superficial (cutaneous) cervical plexus (Fig. 35-6) and traverses cephalad from the posterior edge of the sternocleidomastoid muscle toward the top of the head, dividing into several branches. The greater occipital nerve arises from the posterior ramus of the second cervical spinal nerve (the cervical plexus arises from the anterior rami) and travels in a cranial direction to reach the skin in the area of the superior nuchal line while giving branches to supply the head and laterally toward the ear.
These nerves can be blocked by superficial injection at the point on the posterior skull where they emerge from below the muscles of the neck.
Spine
Spinal/epidural anesthesia is not discussed in this chapter, but a basic description of the spinal nerves as well as vertebral structures is provided, given their relevance to the performance of other regional blocks.
Spinal Nerves
The spinal nerves are part of the peripheral nervous system, along with the cranial and autonomic nerves and their ganglia. There are 31 pairs of spinal nerves—8 cervical (C1–C8), 12 thoracic (T1–T12), 5 lumbar (L1–L5), 5 sacral (S1–S5), and 1 coccygeal.
The spinal nerves are formed by the union of the ventral (anterior) and dorsal (posterior) spinal roots and consist of both motor and sensory fibers. In addition, all spinal nerves contain sympathetic fibers for supplying blood vessels, smooth muscle, and glands in the skin.
The nerves give off sympathetic branches immediately after leaving the intervertebral foramen. Gray and white rami communicantes connect the spinal nerves to the sympathetic chain ganglia to allow preganglionic sympathetic fibers leaving the spinal cord (T1–L2/L3) to enter the chain and leave it again to be distributed with spinal nerves at all levels.
Soon after exiting the intervertebral (spinal) foramina, each spinal nerve in turn divides into a larger ventral and a smaller dorsal ramus (branches). The ventral rami course laterally and anteriorly to supply the muscles, subcutaneous tissues (superficial fascia) and skin of the neck, trunk, and the upper and lower extremities (see the dermatomes of the body in Fig. 35-8). The dorsal rami course posteriorly and supply the paravertebral muscles, subcutaneous tissues, and skin of the back close to the midline.
It is important to realize that the first cervical (C1) nerve leaves the spinal cord and courses above the atlas (C1 vertebra). Hence the cervical nerves are numbered corresponding to the vertebrae inferior to them. From this point on, all the spinal nerves are named corresponding to the vertebral level above. For example, the T3 and L4 spinal nerves exit below the T3 and L4 vertebrae, respectively.
Paravertebral Space
The paravertebral space is a bilateral wedge-shaped area between the individual vertebrae, on either side of and extending the entire length of the vertebral column. The spinal nerves pass through this space, giving off their sympathetic branch and a small dorsal sensory branch before exiting from the intervertebral foramina. In the thoracic region, its boundaries are as follows:
Medially: The vertebral body, intervertebral disc and foramen, and spinous processes (angulation decreases from T1 to L4/L5);
Anterolaterally: The parietal pleura; and
Posteriorly: The costotransverse process, approximately 2.5 cm from the tip of the spinous process, often in a slightly caudad orientation.
The intervertebral foramina at each level lie between the transverse processes and approximately 1 to 2 cm anterior to the plane formed by the transverse processes in their associated fasciae. At this point, the sympathetic ganglia lie close to the somatic nerves, and coincidental sympathetic blockade is usually attained.
Orientation of the Vertebral Body Processes
There are variations in the anatomy of the vertebral column that should be considered when determining the desired location for needle insertion during blocks of the trunk.
The spinous processes lie in the midline, with T7 at the distal tips of the scapulae and L4 at the level of the iliac crests.
The transverse processes lie approximately 2.5 cm lateral to the spinous processes: At T1, the transverse process is directly lateral to its corresponding spinous process, but subsequent transverse processes are extended to increasingly cephalad locations (i.e., T7 transverse process is lateral to T6 spinous process).
In the lumbar region, the spinous processes are straight, and the transverse processes lie opposite their own respective spinous process.
Upper Extremity
Brachial Plexus
The brachial plexus (Fig. 35-10) classically arises from the anterior primary rami of C5–C8 and T1 spinal nerves. The plexus consists of five roots, three trunks, six divisions (two per trunk), three cords, and five major terminal nerves.
The C5–T1 nerve roots emerge from their corresponding intervertebral foramina and then travel along the grooves between the anterior and posterior tubercles of the corresponding transverse process. They finally emerge between the scalenus anterior and medius muscles, above the second part of subclavian artery and posterior to vertebral artery.
The C5 and C6 nerve roots unite to form the upper (superior) trunk, C7 continues as the middle trunk, and C8 and T1 converge into the lower (inferior) trunk.
Fibrous sheaths (as part of the prevertebral fascia) surround the anterior and posterior parts of the plexus and continue to
envelope the plexus between the scalene muscles more distally (called the interscalene fascial sheath proximally and the axillary sheath distally).
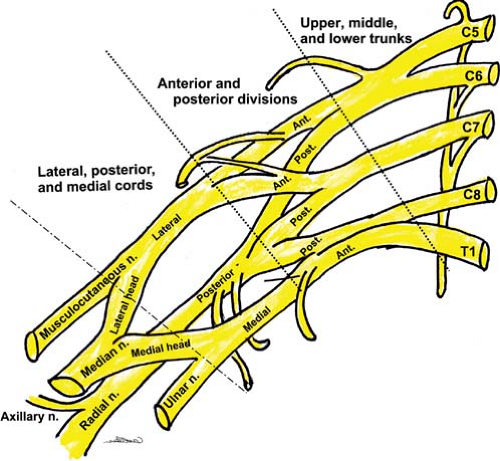
Figure 35-10. Schematic of the brachial plexus. Many branches, including the medial cutaneous nerves of the forearm and arm, which arise from the medial cord are not shown here.
The three trunks travel inferolaterally and cross the base of the posterior triangle of the neck (superficial) and the first rib (upper and middle trunks above the subclavian artery and lower trunk behind or below the artery). At the lateral border of first rib, each trunk bifurcates into anterior and posterior divisions.
Approximately at the level where the nerves course under the pectoralis minor muscle, the divisions converge to form three cords: Lateral cord—anterior divisions of upper and middle trunks (C5–C7); medial cord—anterior division of lower trunk (C8, T1); posterior cord—posterior divisions of all three trunks (C5–T1).
The cords are grouped around the second part of the axillary artery (within 2.5 cm from its center).68 There are three parts of the axillary artery named for their positions above (medial to), behind, and below (lateral to) the pectoralis minor muscle. Typically, with a US probe placed to view the transverse axis of the cords, the medial cord lies inferior, the lateral cord superior, and the posterior cord posterior to the first part of the axillary artery.
Immediately beyond the pectoralis minor muscle, the three cords diverge into the terminal branches; these include the median, ulnar, radial, axillary, and musculocutaneous nerves.
The phrenic nerve normally descends anterior to the scalenus anterior muscle and crosses the muscle from lateral to medial as it descends and passes under the clavicle and through the superior thoracic aperture into the superior mediastinum, just medial to the external jugular vein. However, there is anatomic variation of the course of the phrenic nerve and it is not always anterior to the scalenus anterior muscle.
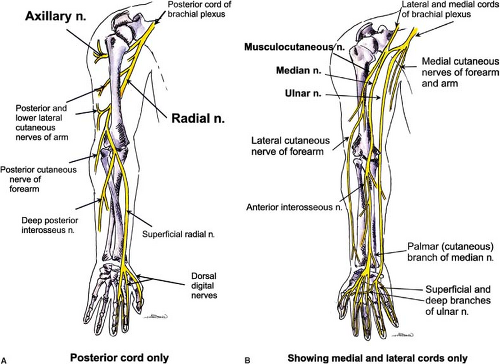
Terminal Nerves of the Brachial Plexus
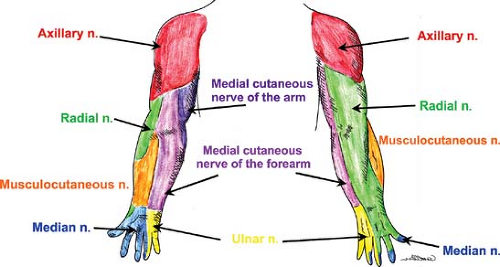
The anatomy of the peripheral nerves is outlined here, although the clinically related innervation patterns are included in the discussion of each block’s technique. Figure 35-11 illustrates the courses of these nerves within the upper extremity. Figure 35-12 illustrates the cutaneous innervation of the terminal nerves of the upper extremity. The axillary nerve is an additional terminal nerve of the upper extremity, but the anatomy and blocking of this nerve will not be discussed here.
Radial Nerve (Originates from C5–C8 and T1 Roots, Upper and Middle Trunks, Posterior Divisions, and Posterior Cord)
This nerve originates deep (often posteromedial)69 to the axillary artery, descends within the axilla (giving off branches to long head of the triceps brachii), passes between the medial and lateral heads of the triceps, and then descends obliquely across the posterior aspect of the humerus along the spiral (radial) groove at the level of the deltoid insertion.
The nerve travels posterior and medial to the deep brachial artery of the arm and reaches the lateral margin of the humerus 5 to 7 cm above the elbow before crossing over the lateral epicondyle and entering the anterior compartment of the arm.
In front of the elbow, the nerve divides and continues as the superficial radial (sensory) and the deep posterior interosseous (motor) nerves.
Median Nerve (Originates from C5–C8, T1, All Trunks, and Lateral and Medial Cords)
In the axilla, this nerve often lies anterolateral to the axillary artery.69,70 The nerve descends along the medial aspect of the arm lateral to the brachial artery and crosses the artery, usually anteriorly, at the midpoint of the arm at the insertion of the coracobrachialis muscle.
The nerve crosses the elbow lying medially on the brachialis muscle and just medial to the brachial artery and vein (all of these are medial to the biceps brachii tendon).
Distal to the antecubital fossa, the nerve gives off the anterior interosseous nerve and cutaneous sensory branches.
Musculocutaneous Nerve (Originates from C5–C7 Roots, Upper and Middle Trunks, Anterior Divisions, Lateral Cord)
This nerve leaves the fascial sheath of the plexus approximately at the level of the coracoid process; thus, the infraclavicular location for brachial plexus block is the most distal block for this nerve.
Just distal (2 to 3 cm) to the pectoralis major muscle attachment, the nerve usually pierces the coracobrachialis muscle, after which it exits and comes to lie between the coracobrachialis muscle and the short and long heads of the biceps brachii muscle.
Although it is difficult to observe using US, the nerve continues as the lateral cutaneous nerve of the forearm at the antecubital fossa and courses along the lateral aspect of the forearm providing subsequent anterior and posterior branches.
Ulnar Nerve (Originates from C7–C8, T1 Roots, Lower Trunk, Anterior Division, Medial Cord)
Initially, the nerve often courses between the axillary artery and vein (it may lie anteromedial to the artery and vein) and then along the medial aspect of the brachial artery to the midpoint of the humerus before passing posteriorly and following the anterior surface of the medial head of the triceps.
The nerve then passes behind the medial epicondyle of the humerus (in the condylar groove), divides between the humeral and ulnar heads of the flexor carpi ulnaris, and lies on the medial aspect of the elbow joint.
During its descent through the forearm, the nerve courses anteriorly, to approach the ulnar artery directly anterior to the ulna at the junction of the lower third and upper two-thirds of the forearm.
At the wrist, it crosses superficial to the flexor retinaculum and divides into superficial and deep branches; the ulnar artery lies anterolateral to the nerve at the wrist.
Anatomic Variation
There are many variations in the anatomy of the brachial plexus71 and in the course of the terminal nerves and vascular elements. Some of these variations may contribute to difficulty when performing PNB, since there may be unexpected NS responses (e.g., if two nerves are conjoined) or poor localization by NS or by US imaging (e.g., if the nerve follows a substantially different path). Some examples are described below:
The plexus may include anterior rami from C4 to C8 (“prefixed”) or, less commonly, from C5 to T2 (“postfixed”).
The existence and/or characteristics of the connective tissue sheath that invests the plexus at various regions are controversial. A continuous, tubular sheath has been shown unlikely, especially in the axillary region. A more convoluted and septated structure may be the cause of nonuniform distribution of local anesthetic in many cases, which supports the findings that multiple injection techniques may be superior.72 US guidance can be valuable in this location to ensure circumferential spread of local anesthetic around the nerves.
The interscalene groove may have variation in the relationship between the plexus roots and trunks and the muscles. For example, the C5 and/or C6 nerve roots may traverse either through or anterior to the anterior scalene muscle.73
In many cadaver specimens, no inferior trunk exists.74 A single cord or a pair of cords may develop. It has been observed that no discrete posterior cord forms in some cases, with the posterior divisions diverging to form terminal nerves.71
The terminal nerves may lie in various relations to the axillary vessels. The use of combined NS- and US-guided technique to both confirm the nerve localization (NS) and obtain circumferential spread of local anesthetic around each of the nerves (US) may improve block success.8 The musculocutaneous nerve may fuse to or have communications with the median nerve, which can result in the absence of the former from within the coracobrachialis muscle.75,76 Communication between the median and ulnar nerves in the forearm are common, with the median nerve replacing the innervation to various muscles normally supplied by the ulnar nerve.77
Trunk
Intercostal Nerves and Articulations
Intercostal Nerves
At the thoracic level, each anterior primary ramus enters a neurovascular bundle with its respective artery and vein and travels along the intercostal groove on the ventral caudad surface of each rib.
The fasciae of the internal and external intercostal muscles provide interior and external borders of the intercostal groove.
As the intercostal nerves travel beyond the midaxillary line, they give off a lateral sensory branch, while the main trunk continues on to the anterior abdominal wall to provide sensory and motor innervation for the trunk and abdomen down to the level of the pubis.
The intercostal groove becomes much less well defined anterior to the midaxillary line, and the nerves begin to move away from their protected position. The lowermost intercostal nerve (subcostal; the 12th) is much less proximal to its accompanying rib and is not as easy to identify and anesthetize using a classic intercostal blockade technique.
Costovertebral Articulations
The ribs articulate through two synovial joints with the vertebral column, each enclosed in fibrous capsules that are reinforced by ligaments:
Costovertebral joint is a synovial articulation of the head of the rib with the demi-facets on the adjacent thoracic vertebral bodies and the corresponding intervertebral disc of the upper vertebral joint (except for 1st, 10th, 11th, and 12th ribs, which articulate with a single vertebral facet).
Costotransverse joint is a synovial joint between the articular facets on the tubercles of the ribs and the transverse processes of the thoracic vertebrae (the 11th and 12th ribs lack this articulation since they do not possess tubercles). Penetration of the costotransverse ligament may occur during paravertebral block.
Lumbar Spinal Nerves and Plexus
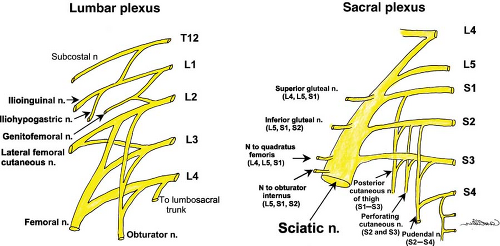
The spinal nerves at the lumbar level follow the same course as those of the thoracic level when leaving the intervertebral foramen, yet the anterior (ventral) rami form the lumbar plexus instead of continuing as intercostal nerves. The lumbar plexus (Fig. 35-13) is formed by the union of the anterior primary rami of L1–L3 and part of L4.
The upper nerve roots emerge from their foramina into a compartment lined by the fasciae of muscles anterior and posterior to it. In this case, the quadratus lumborum is posterior, while the posterior fascia of the psoas muscle provides the anterior border of the compartment before the nerves move into the body of the muscle.
The lumbar plexus supplies the skin and muscles of the lower part of the anterior abdominal wall (including the external genitalia) and the skin and muscles of the anterior and medial
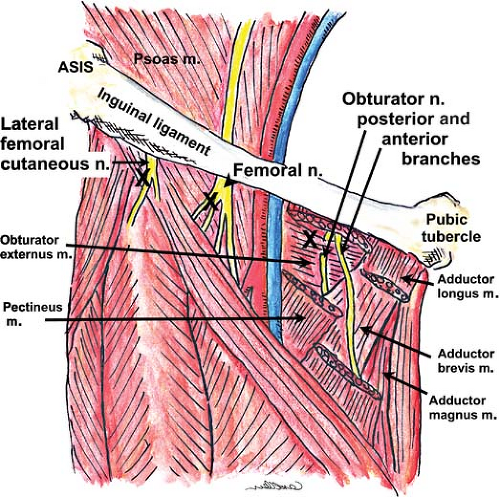
compartments of the thigh. L1 bifurcates into an upper part (iliohypogastric and ilioinguinal nerves) and a lower part, which joins with a branch from L2 to form the genitofemoral nerve. L3, with portions of L2 and L4, divides into anterior and posterior divisions; the anterior division forms the obturator (L2–L4) and accessory obturator (L3, L4, when present) nerves, and the posterior division forms the lateral (femoral) cutaneous nerve of the thigh (L2–L3) and the femoral nerve (L2–L4).
In anatomic relation to the psoas major muscle, the obturator (L2–L4) and accessory obturator nerves emerge from its medial border, the genitofemoral (L1, L2) pierces the muscle to lie on its anterior surface, and all others emerge from its lateral border.
Terminal nerves of the lumbar plexus are discussed in the Lower Extremity section.
Inguinal Nerves
The iliohypogastric nerve penetrates the transverse abdominis muscle just above the iliac crest, supplies it, and divides into anterior and lateral cutaneous branches:
The anterior branch pierces and supplies the internal oblique muscle just 2 cm medial to the anterior superior iliac spine. It then courses deep to the external oblique muscle and superior to the inguinal canal and pierces the external oblique aponeurosis about 2 to 3 cm above the superficial inguinal ring, terminating subcutaneously in the skin of the suprapubic region.
The lateral cutaneous branch supplies the anterolateral portion of the gluteal skin after piercing both the oblique muscles. The ilioinguinal nerve pierces and supplies the internal oblique muscle and then enters the inguinal canal, in which it traverses outside the spermatic cord to emerge through the superficial (external) inguinal ring (the external oblique aponeurosis), where it provides cutaneous innervation to the skin of the scrotum (or labium majus) and adjacent thigh.
Lower Extremity
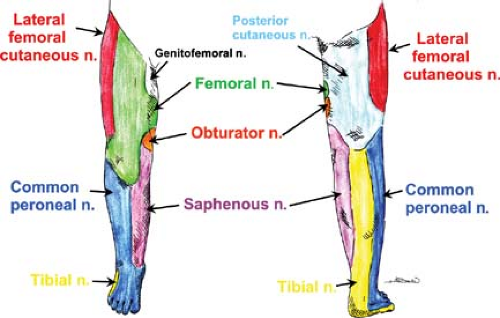
Together, the lumbar and sacral plexuses (Fig. 35-13) supply the lower limb. The formation of the lumbar plexus is discussed in the section above. Important landmarks that contain the plexus during its course include the psoas compartment, bordered posteriorly by the quadratus lumborum muscle and anteriorly by the posterior fascia of the psoas muscle, and, more distally, the substance of the psoas major muscle. The anatomy of the terminal nerves is examined below, as are the formation and branches of the sacral plexus. The cutaneous innervation in the lower extremity is shown in Figure 35-14. The lower extremity dermatomes are shown in Figure 35-8.
Sacral Plexus: Formation and Branches
At the medial border of the psoas major muscle, the lumbosacral trunk is formed by the union of a branch of L4 and the anterior ramus of L5. After exiting through the anterior sacral foramina, the anterior primary rami of S1–S4 join the lumbosacral trunk to form the sacral plexus (Fig. 35-13). The nerves of the plexus converge toward the greater sciatic foramen anterior to the piriformis muscle on the posterior pelvic wall. The main terminal nerves are the sciatic nerve (continuation of the plexus) and the pudendal nerves (“terminal branches”). Several other small branches are given off, including muscular branches (e.g., inferior and superior gluteal nerves and nerves to the quadratus femoris, piriformis, obturator internus, and external sphincter muscles), cutaneous branches (e.g., posterior cutaneous nerve of the thigh), and visceral branches (pelvis splanchnic nerves). The gluteal vessels (superior and inferior) generally follow the course of the sacral nerves in the anterior plane and can be used to help identify the sciatic nerve at its proximal course. Additional vascular structures that may be identified under US imaging are the pudendal vessels, which pass from the greater to lesser sciatic foramen between the sciatic and pudendal nerves.
Sciatic, Tibial, and Common Peroneal Nerves
The sciatic nerve—the largest nerve of the body—is usually the conjunction of two trunks initially enveloped in a common sheath: A lateral trunk (L4–S2), which eventually emerges as the common peroneal nerve and a medial trunk (L4–S3), which later becomes the tibial nerve. These combined nerves exit through the sciatic notch and pass anteriorly to the piriformis muscle to then lie between the ischial tuberosity and the greater trochanter of the femur. They curve caudally and descend in the posterior thigh adjacent to the femur. At a variable distance within the posterior thigh (often high in the popliteal fossa), the sciatic nerve bifurcates into the tibial and common peroneal nerves. The common peroneal nerve descends along the medial border of the biceps femoris muscle and then on the lateral border of the gastrocnemius muscle. At the fossa it gives off the lateral sural nerve, which forms the lateral sural cutaneous nerve by joining the medial sural nerve supplied by the tibial nerve. It winds around neck of the fibula and terminates as the deep and superficial peroneal nerves. In the posterior thigh, the tibial nerve is covered medially by the semitendinosus and semimembranosus muscles and laterally by the biceps femoris muscle. Beyond the knee joint, it is covered by both heads of the gastrocnemius muscle and then deep to the soleus muscle, before coming to an end on the tibialis posterior muscle and finally on the posterior surface of the tibial shaft medial to the medial malleolus. Within the fossa, it gives off muscular branches (gastrocnemius, soleus, popliteus, and plantaris muscles) as well as the medial sural nerve (to join its lateral counterpart from the common peroneal nerve). In the lower leg and foot, it gives off muscular, articular (ankle), and cutaneous branches and terminates as the medial and lateral plantar nerves. The nerve is often called the posterior tibial nerve in the lower leg.
Terminal Nerves of the Lumbar Plexus
Genitofemoral Nerve (L1, L2)
This nerve leaves the lumbar plexus at the lower border of the L3 vertebra. It pierces and then lies anterior to the psoas major muscle before descending subperitoneally and behind the ureter, where it divides into two branches (genital and femoral) at a variable distance above the inguinal ligament. The genital branch crosses the external iliac artery and traverses the inguinal canal. It supplies the cremaster muscle and skin over the scrotum and adjacent thigh (males) or the skin over anterior part of labium majus and mons pubis (females). The femoral branch descends lateral to the external iliac artery, passes under the inguinal ligament, enters the femoral sheath lateral to the femoral artery, and pierces the anterior layer of the femoral sheath and fascia lata. It innervates the skin immediately below the crease of the groin anterior to the upper part of the femoral triangle.
Lateral Cutaneous Nerve of Thigh (aka, Lateral Femoral Cutaneous Nerve) (L2, L3)
This nerve passes obliquely from the lateral border of the psoas major muscle over the iliacus to enter the thigh below or through the inguinal ligament, variably medial to the anterior superior iliac spine (Fig. 35-15). On the right side of the body, the nerve passes posterolateral to the cecum, and on the left it traverses behind the lower part of the descending colon. The nerve lies on top of the sartorius muscle before dividing into anterior (supplies skin over the anterolateral aspect of the thigh) and posterior (supplies skin on the lateral aspect of thigh from the greater trochanter to the midthigh) branches. Occasionally, this nerve is a branch of the femoral nerve rather than its own nerve.
Femoral Nerve (L2–L4)
The femoral nerve is the largest nerve of this plexus, supplying muscles and skin on the anterior aspect of the thigh. It descends through the psoas major muscle and emerges low at its lateral border, coursing inferiorly between the iliacus and psoas major muscles to enter the thigh under the inguinal ligament (Fig. 35-15). At the inguinal ligament (line running between anterior superior iliac spine and the medial pubic tubercle) and just distal to it (in the femoral triangle), the nerve lies slightly deeper (0.5 to 1 cm) and lateral (approximately 1.5 cm) to the femoral artery; the vein is medial to the artery (“VAN” is the mnemonic for the anatomical relationship, starting medially). At the femoral (inguinal) crease (a few centimeters caudad to the inguinal ligament), the nerve lies underneath the fascia iliaca (iliopectineal fascia), deep to the fascia lata. Beyond the femoral triangle, the nerve branches into anterior (quite proximally) and posterior divisions. The anterior division gives muscular branches to the pectineus and sartorius muscles and cutaneous branches (intermediate and medial cutaneous nerves of thigh) to the skin on the anterior aspect of the thigh. The posterior division sends muscular branches to the quadriceps femoris muscle and gives rise to the saphenous nerve, its largest cutaneous branch. The saphenous nerve follows the femoral artery, lying lateral to it within the adductor (Hunter’s, subsartorial) canal and then crossing it anteriorly to lie medial to the artery. Distal to the canal, the saphenous nerve leaves the artery to lie superficial at the medial aspect of the knee; the nerve then continues inferiorly (subcutaneously) with the long (great) saphenous vein along the medial aspect of the leg down to the tibial aspect of the ankle. The saphenous branch supplies the skin on the medial aspect of the leg below the knee and on the medial aspect of the foot; it provides articular branches to the hip, knee and ankle joints.
Obturator Nerve (L2–L4)
The obturator nerve emerges from the medial border of the psoas major muscle at the pelvic brim to pass behind the common iliac vessels and lateral to the internal iliac vessels. It then courses inferiorly and anteriorly along the lateral wall of the pelvic cavity on the obturator internus muscle toward the obturator canal,
through which it enters the upper part of the medial aspect of the thigh above and anterior to the obturator vessels. The nerve divides into its anterior and posterior branches near the obturator foramen (Fig. 35-15); the anterior branch passes into the thigh anterior to the obturator externus, descends in front of the adductor brevis, and behind the pectineus and adductor longus muscle, with its terminal cutaneous branches emerging as it courses alongside the femoral artery. It supplies the adductor longus, gracilis, adductor brevis (usually), and pectineus (often) muscles. Cutaneous branches supply the skin on the medial aspect of the thigh and perhaps to the medial knee. The nerve’s posterior branch pierces the obturator externus muscle anteriorly and supplies it, then passes behind the adductor brevis muscle (sometimes supplies it) to descend on the anterior aspect of the adductor magnus muscle (medial to the anterior branch), which it supplies. There is no apparent cutaneous supply from this nerve. It then traverses the adductor canal with the femoral artery and vein to enter the popliteal fossa, where it terminates as an articular branch to the back of the knee joint capsule (oblique popliteal ligament).
through which it enters the upper part of the medial aspect of the thigh above and anterior to the obturator vessels. The nerve divides into its anterior and posterior branches near the obturator foramen (Fig. 35-15); the anterior branch passes into the thigh anterior to the obturator externus, descends in front of the adductor brevis, and behind the pectineus and adductor longus muscle, with its terminal cutaneous branches emerging as it courses alongside the femoral artery. It supplies the adductor longus, gracilis, adductor brevis (usually), and pectineus (often) muscles. Cutaneous branches supply the skin on the medial aspect of the thigh and perhaps to the medial knee. The nerve’s posterior branch pierces the obturator externus muscle anteriorly and supplies it, then passes behind the adductor brevis muscle (sometimes supplies it) to descend on the anterior aspect of the adductor magnus muscle (medial to the anterior branch), which it supplies. There is no apparent cutaneous supply from this nerve. It then traverses the adductor canal with the femoral artery and vein to enter the popliteal fossa, where it terminates as an articular branch to the back of the knee joint capsule (oblique popliteal ligament).
Accessory Obturator Nerve (L3, L4)
This nerve is present in about 30% of individuals. It descends along the medial border of the psoas major muscle, crosses the superior pubic ramus behind the pectineus muscle, supplies it, and gives articular branches to the hip joint.
Nerves at the Ankle
By the time the femoral, tibial, and common peroneal nerves reach the ankle, there are five branches that cross this joint to provide innervation for the skin and muscles of the foot.
Deep Peroneal Nerve (L5, S1)
This nerve lies anterior to the tibia and interosseus membrane and lateral to the anterior tibial artery and vein at the ankle. It travels deep to and between the tendons of the extensor hallucis longus and extensor digitorum longus muscles. Beyond the extensor retinaculum, it branches into medial and lateral terminal branches; the medial branch passes over the dorsum of the foot and supplies the first web space through two terminal digital branches, and the lateral branch traverses laterally and terminates as the second, third, and fourth dorsal interosseus nerves.
Tibial Nerve (aka, Posterior Tibial Nerve; S1–S3)
On the posterior aspect of the knee joint, the tibial nerve joins the posterior tibial artery and then runs deep through to the lower third of the leg where it emerges at the medial border of the calcaneal tendon (Achilles tendon). Behind the medial malleolus it lies beneath several layers of fascia and is separated from the Achilles tendon only by the tendon of the flexor hallucis longus muscle. The nerve is posteromedial to the posterior tibial artery and vein, which are, in turn, posteromedial to the tendons of the flexor digitorum longus and tibialis posterior muscles. Just below the medial malleolus, the nerve divides into the lateral and medial plantar nerves. The nerve innervates the ankle joint through its articular branches and the skin over the medial malleolus, the inner aspect of the heel (including Achilles tendon), and the dorsum of the foot (through the medial and lateral plantar nerves) with its cutaneous branches.
Superficial Peroneal Nerve
Sural Nerve
This nerve arises from tibial (medial sural nerve) and common peroneal (lateral sural nerve) nerves. It emerges to the superficial compartment at a similar but posterior level to the superficial peroneal nerve, 7 to 8 cm above the lateral malleolus. It then curves around the malleolus at some distance (1 to 1.5 cm) to enter and innervate the lateral aspect of the dorsal surface of the foot.
Saphenous Nerve
The saphenous nerve is the superficial terminus of the femoral nerve, which supplies the skin over the lower medial leg (Fig. 35-14). It leaves the femoral nerve proximally in the femoral triangle (Scarpa’s triangle), descends within the adductor canal, and courses beneath the sartorius muscle with the femoral artery (beginning lateral of the vessel at first and then crossing to the medial side superior to the artery just proximal of the lower end of the adductor magnus muscle). Further distally, the femoral artery departs away from the sartorius muscle, traveling deep to continue as the popliteal artery at the adductor hiatus. At this location, the saphenous nerve continues its course under the sartorius muscle, traveling adjacent to the saphenous branch of the descending genicular artery. It runs superficial at the medial surface of the lower leg and in front of the heel.
Specific Techniques
The remainder of this chapter is devoted to the procedural details of specific blocks, arranged, similar to the above section on anatomy, by regions of the body. In the sections for Upper Extremity, Trunk, and Lower Extremity, details for using NS and US imaging during the blocks are included. The nerve stimulator is set to deliver variable currents with a frequency of 2 Hz and pulse width of 0.1 ms unless stated otherwise. The volumes of local anesthetic included are those suggested for blocks during which NS was used for nerve localization; US guidance may reduce the required volume in some instances. The figures in these sections will focus predominantly on using combined US and NS-guided technique, although procedures for blind techniques using NS are also described. It is important to note that the figures illustrating technique in humans are representative of the clinical scenario, but without all of the sterile preparation required so as to facilitate observation of proper probe and needle handling. The description of each technique is accompanied by practical tips and evidence-based recommendations. In addition, most of the suggestions related to volume of local anesthetic are based on conventional technique. Although it is not yet well established, many experts speculate that the use of US guidance may reduce the volume of local anesthetic required to achieve adequate blockade.
Head and Neck
Regional anesthesia for the head and neck is diverse, and many head and neck surgical procedures are amenable to some form of regional block. A regional technique may be the sole mode of anesthesia or may be incorporated into a balanced general anesthetic offering optimal postsurgical analgesia. Blocks can be used for ophthalmic, neurologic, ENT, plastic, and endocrine surgeries. Regional anesthesia techniques, such as trigeminal or occipital nerve block, may also be used for diagnostic and therapeutic purposes in acute and chronic pain syndromes. Block techniques range from local infiltration to field block to specific nerve blocks. Since intraoperative airway control can be challenging, the absence of definitive airway control is a frequent source of concern with regional techniques.
Regional anesthesia of the head and neck depends primarily on local infiltration and/or specific nerve blocks placed with reliable anatomical landmarks. Elicitation of a paresthesia is the mainstay of nerve localization, while neither NS nor US imaging have been performed or reported to any extent for these blocks. Therefore, the description of techniques in this section will deviate from other areas where there is greater reliance on nerve localization modalities using NS and US imaging.
Trigeminal Nerve Blocks
For every procedure, prepare the needle insertion site and other applicable skin areas with an antiseptic solution and use sterile equipment. All of the blocks described below use the extraoral route, although alternative intraoral routes may be suitable in many cases.
Semilunar (Gasserian) Ganglion Block
The most comprehensive blockade of the trigeminal nerve targets the central ganglion (Fig. 35-4). This block is usually performed by neurosurgeons under fluoroscopic guidance for treatment of disabling trigeminal neuralgia. Few anesthesiologists perform this technically difficult block and it will not be described in detail here.
Superficial Trigeminal Nerve Branch Block
Trigeminal block can be easily performed by injection of the three individual terminal superficial branches (supraorbital, infraorbital, mental nerves). Each nerve is closely associated with their respective foramina, and all foramina lie in the same sagittal plane on each side of the face (approximately 2.5 cm lateral to the midfacial line passing through the pupil) (Fig. 35-16) and are easily located by US.81 These foramina are readily palpable, and these nerves can be blocked with superficial injections of small quantities of local anesthetic. The bony landmarks are usually sufficient themselves for routine anesthetic purposes. However, paresthesias are desirable when performing neurolytic blocks with alcohol. An additional block of the supratrochlear nerve is required if the field of anesthesia is to cross the midline (Fig. 35-5). Generally, fine, short needles (e.g., 24 to 26 gauge, 25 to 40 mm) and small syringes (1 to 5 mL) will be suitable for these blocks. The block is usually performed with the patient in the supine position.
Procedure
Supraorbital nerve (terminal nerve of ophthalmic branch). The supraorbital notch is easily palpated at the medial upper angle of the orbit or located by US as shown in Figure 35-16. The needle is inserted and local anesthetic (see Comments) is slowly injected after aspiration, slightly outside the notch, and produces anesthesia of the ipsilateral forehead.
Supratrochlear nerve (terminal nerve of ophthalmic branch). Anesthesia of the supratrochlear nerve is obtained with
superficial infiltration of the upper internal angle of the orbital rim. This is needed if the field of anesthesia is to cross the midline.
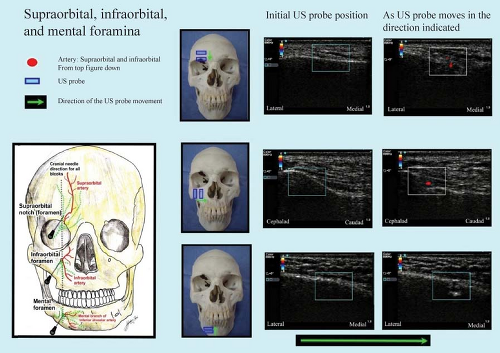
Figure 35.16. US showing the supraorbital, infraorbital, and mental foramena. The discontinuation of the hyperechoic bony line indicates a gap along the bone surface which is the foramen.
Infraorbital nerve (terminal branch of maxillary nerve). The infraorbital foramen lies about 1 cm below the middle of the lower orbital margin. If the foramen cannot be palpated directly, it can be identified by locating the discontinuity of the hyperechoic line on US81 (Fig. 35-16). The needle should be introduced in a cranial direction through a skin wheal approximately 0.5 cm below the expected opening. After making contact with the bone and withdrawing slightly, injection of a small quantity of local anesthetic is performed. This block produces anesthesia of the middle third of the ipsilateral face.
Mental nerve (sensory terminal branch of mandibular nerve). The mental nerve emerges from its foramen, which lies inferior to the outer lip at the level of the second premolar, midway between the upper and lower borders of the mandible. The mental canal angles medially and inferiorly; in this case, therefore, needle insertion should start approximately 0.5 cm above and 0.5 cm lateral to the anticipated location of the orifice if it cannot be palpated directly. Again, the use of US can aid identification of the foramen (Fig. 35-16). Slow injection after aspiration at the opening of the canal produces anesthesia of the mandibular area. Injection directly into the canal should be avoided to reduce the risk of neural injury.
Comments
Choice of local anesthetic for all blocks will depend on the purpose of the block and the duration of anesthesia required (e.g., 1% mepivacaine for shorter procedures and 0.75% ropivacaine for longer procedures). For surgical anesthesia, 2 to 5 mL of local anesthetic may be used, while diagnostic or therapeutic volumes will be much smaller (0.5 to 1 mL).
The blocks should be followed by local compression to prevent hematoma formation.
PNB of the terminal branches of the trigeminal nerve offers a safe and effective alternative to local infiltration for soft-tissue injury of the face. Despite this, local infiltration is often required to rectify incomplete anesthesia, especially of the supraorbital and infraorbital nerves.82
Infraorbital nerve block may be performed for postoperative analgesia after cleft lip repair. Palpating anatomic landmarks for this block can be difficult in the neonate, due to the developing facial configuration.
Skull nerve blocks can be used for craniotomy procedures and are also recommended to attenuate postoperative pain.83 The nerves blocked to achieve successful anesthesia for craniotomy include the supraorbital and supratrochlear nerves, the greater and lesser occipital nerves, the auriculotemporal nerves and the greater auricular nerves.
Supraorbital nerve blocks have been associated with a high requirement for supplementation, perhaps due to the anatomic
variation of the nerve. The nerve may exit the skull undivided or its medial and lateral branches may exit separately. For frame pin placement during stereotactic neurosurgery, failure to block the lateral branch may account for inadequate coverage.84
During mental nerve block in older patients, resorption of the superior margin of the mandible will make the foramen appear to lie more superiorly along the ramus.
Maxillary Nerve Block
This block should be performed by practitioners with related and adequate experience. It is required when superficial block of the infraorbital nerve does not produce adequate anesthesia or when anesthesia of the more proximal superior dental nerves is required. This can be performed by a lateral approach to the sphenopalatine fossa.
Procedure
The patient either sits with the mouth slightly open, or lies supine with a small towel under the occiput and the head turned slightly away from the side to be blocked.
Above the zygomatic arch: The center of the upper zygomatic arch is marked. A 60- to 90-mm needle is introduced at 45 degrees, caudally and medially, toward the contralateral molar teeth. After a paresthesia is elicited at the nostril, upper lip and cheek, slow incremental injection of local anesthetic is performed after slight needle withdrawal and with frequent aspiration.
Below the zygomatic arch (Fig. 35-17): The zygomatic arch is marked along its course, and the patient is asked to open and close the mouth slowly so that the curved upper border of the mandible can be identified. The mandibular fossa is palpated between the condylar and coronoid processes. The lowest point of the mandibular notch is palpated, and an “X” is marked at this spot, which is usually at the midpoint of the zygoma. A local anesthetic skin wheal is raised at the “X” after appropriate skin preparation.
With the patient’s jaw in the open position, a 60- to 90-mm needle is introduced through the “X” at a 45-degree angle toward the dorsal part of the eyeball (cephalad and slightly anterior).
The needle should contact the lateral portion of the pterygoid process (pterygoid plate) at a depth of 4 to 5 cm. It is then withdrawn and redirected slightly cephalad and anteriorly until it passes beyond the pterygoid plate and enters the pterygopalatine fossa at an additional depth of no more than 1 cm. A paresthesia in the nose or the upper teeth confirms nerve localization. The pterygopalatine fossa is highly vascular, so care must be exercised to avoid intravascular injection.
Anesthesia can be achieved by injecting 5 mL into the pterygopalatine fossa, either on obtaining the paresthesia or blindly by advancing 1 cm beyond the plate.
Comments
One concern during this block is spread of local anesthetic to adjacent structures, especially to the nerves in the orbit. If pain occurs in the region of the orbit during the procedure, the injection should be stopped and the needle should be withdrawn.
Although the mainstay of treatment for trigeminal neuralgia continues to be pharmacologic or neuroabalative, maxillary nerve block with extraoral mandibular nerve block has been reported to provide relief in some settings.85
Mandibular Nerve Block
This nerve can be blocked for dental and maxillary surgery or for inferior dental pain, trigeminal neuralgia in the third branch, or temporomandibular joint dysfunction. It is the only branch of the trigeminal nerve where anesthesia carries the risk of loss of motor (mastication) function.
Procedure
The patient lies supine with the face in profile. Landmarks for location of the mandibular fossa are the same as those described for maxillary nerve blockade.
A 60- to 90-mm needle is introduced through the skin wheal and directed perpendicularly to the skin, without the cephalad angulation required for maxillary nerve anesthesia.
When the pterygoid plate is contacted, the depth should be noted. The needle is then redirected posteriorly until it passes beyond the pterygoid plate. It should contact the nerve 0.5 to 1 cm deep from the point where the pterygoid plate was contacted (Fig. 35-17).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree