PH is defined as a persistent increase in MPAP ≥ 25 mmHg, measured by right heart catheterization (RHC) at rest, coupled with PCWP ≤ 15 mmHg and PVR >240 dyn s/cm5 or interchangeably >3 WU [4–8]. PH patients with left-sided heart disease and who frequently present with PCWP > 15 mmHg are the exception [9]. Classification of PH severity (mild, moderate, and severe) based on the three parameters of PVR, MPAP, and transpulmonary gradient [TPG = MPAP − PCWP] is shown in Table 15.1. In a large cohort study by Vakil et al. on data from the United Network for Organ Sharing (UNOS) database, PVR and TPG were well-correlated (r = 0.85) but the correlation of MPAP with PVR and TPG was only modest. More importantly, PVR and TPG were shown to provide more accurate information than MPAP on the degree of vascular resistance [4]. RHC is considered the gold standard in the diagnosis of PH [5, 6]. In addition to pulmonary artery systolic pressure (PASP), RHC provides information regarding left atrial pressure, PCWP, left ventricular end diastolic pressure, TPG, and PVR.
Table 15.1
Classification of pulmonary hypertension severity based on different definitions
Definition | None | Mild | Moderate | Severe |
|---|---|---|---|---|
Pulmonary vascular resistance (Wood units) | <2.5 | 2.5–3.4 | 3.5–4.9 | ≥5.0 |
Transpulmonary gradient (mmHg) | <13 | 13–16 | 17–19 | ≥20 |
Mean pulmonary artery pressure (mmHg) | <25 | 25–34 | 35–44 | ≥45 |
Transthoracic echocardiography (TTE) has been validated as an important tool for screening and follow-up studies in conjunction with RHC for the assessment of PH patients [7]. It is important to note that TTE cannot be used interchangeably with RHC, the gold standard for definite PH diagnosis [5]. Right ventricular systolic pressure (RVSP) is measured by Doppler echocardiography using peak velocity of tricuspid regurgitation (TR) jet (Fig. 15.1). A simplified Bernoulli equation is utilized in the calculation of RVSP from TR velocity jet.
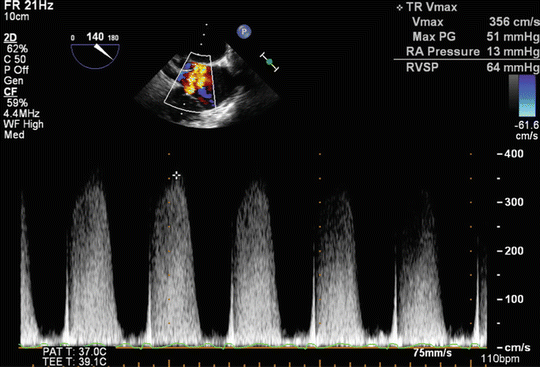
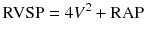 V = Peak velocity of regurgitant jet across tricuspid valve
V = Peak velocity of regurgitant jet across tricuspid valve

Fig. 15.1
Measurement of right ventricular (RV) systolic pressure from tricuspid regurgitation (TR) jet
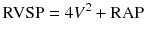
RAP = Right atrial pressure
The caveat is that the accuracy of echocardiographic estimation of RVSP is operator-dependent and limited by feasibility of obtaining reproducible TR jet velocities. In the absence of pulmonic valve stenosis and RV outflow obstruction, PASP is assumed to equate with RVSP. Additionally, TTE is a proven useful noninvasive guide in the evaluation of ventricular size and function, valvular abnormalities, and intra-cardiac shunts.
RAP and right ventricular end diastolic pressure can be quantified during RHC or noninvasively using echocardiography through measurement of inferior vena cava (IVC) diameter or IVC collapse index [8].
Pathophysiology and Classification of Pulmonary Hypertension
Chronic hypoxemia, inflammatory and vascular mediators, and increased shear forces secondary to pathological increases in cardiac output or venous back-pressure can result in impairment of endothelial nitric oxide (NO) synthase and cyclooxygenase activity. Decreased availability of endothelial-derived NO and prostacyclin (PGI2) and increased production of thromboxane A2, endothelin-1 (ET-1), angiotensin-2, and superoxide radicals result in pulmonary vasoconstriction, vascular smooth muscle proliferation, and platelet aggregation. Endothelial damage along with platelet aggregation leads to in situ thrombosis, which ultimately culminates in the formation of plexiform lesions, irreversibly obliterating pulmonary arterioles [9–15].
Patients included in groups 1, 3, 4, and 5 in the World Health Organization (WHO) classification of PH, although of different etiologies, are all characterized by endothelial dysfunction in the pulmonary vasculature (Table 15.2). What ensues is vascular remodeling and histopathological abnormalities, which include intimal and adventitial proliferation, smooth muscle hypertrophy, fibrosis, and plexiform and thrombotic lesions in distal pulmonary arteries [16, 17]. The increase in PVR and elevation in PAP without an increase in PCWP is pathognomonic for patients in groups 1, 3, 4, and 5. Group 2 patients in the WHO PH classification (PH secondary to pulmonary venous hypertension) constitute the majority of PH patients. In post-capillary PH (class 2 WHO), prolonged, untreated elevation of left ventricular end diastolic pressure can result in passive backpressure in the pulmonary veins, which ultimately leads to raised PAP. Vasoconstriction ensues with time, culminating in vascular remodeling of pulmonary arterioles. In contrast to groups 1, 3, 4, and 5, group 2 PH patients may not demonstrate pathophysiologic changes evident in groups 1, 3, 4, and 5. Also, RHC reports for group 2 patients would indicate PCWP values greater than 15 mmHg. Obviously, there is a spectrum of clinical presentations in group 2 patients, evident by PVR in ranging from <240 to >240 dyn s/cm5. This phenomenon correlates with degree of reversibility of PH. High output states constitute another important class of PH patients as PAP = Left atrial pressure + (Cardiac output × PVR)/80. Conversely, in the clinical setting of increased cardiac output caused by inotropes or increased blood volume, PVR will decrease via enrollment of open vessels in the pulmonary circulation [18]. Because of disparate underlying pathophysiology, medical management and perioperative care for group 2 patients fundamentally differ from those designed for group 1, 3, 4, and 5 patients.
Table 15.2
World Health Organization (WHO) updated clinical classification of pulmonary hypertension [8]
1.1. Idiopathic PAH |
1.2. Heritable |
1.2.1. BMPR2 |
1.2.2. ALK1, endoglin, SMAD9, CAV1, KCNK3 |
1.2.3. Unknown |
1.3. Drug- and toxin-induced |
1.4. Associated with |
1.4.1. Connective tissue diseases |
1.4.2. HIV infection |
1.4.3. Portal hypertension |
1.4.4. Congenital heart diseases |
1.4.5. Schistosomiasis |
1′. Pulmonary veno-occlusive disease and/or pulmonary capillary hemangiomatosis |
1″. Persistent pulmonary hypertension of the newborn |
2. Pulmonary hypertension due to left heart disease |
2.1. Left ventricular systolic dysfunction |
2.2. Left ventricular diastolic dysfunction |
2.3. Valvular disease |
2.4. Congenital/acquired left heart inflow/outflow tract obstruction and congenital cardiomyopathies |
3. Pulmonary hypertension due to lung disease and/or hypoxia |
3.1. Chronic obstructive pulmonary disease |
3.2. Interstitial lung disease |
3.3. Other pulmonary diseases with mixed restrictive and obstructive pattern |
3.4. Sleep-disordered breathing |
3.5. Alveolar hypoventilation disorders |
3.6. Chronic exposure to high altitudes |
3.7. Developmental abnormalities |
4. Chronic thromboembolic pulmonary hypertension |
5. Pulmonary hypertension with unclear multifactorial mechanisms |
5.1. Hematological disorders: chronic hemolytic anemia, myeloproliferative disorders, splenectomy |
5.2. Systemic disorders: sarcoidosis, pulmonary histiocytosis, lymphangioleiomyomatosis |
5.3. Metabolic disorders: glycogen storage disease, Gaucher’s disease, thyroid disorders |
5.4. Others: tumor, obstruction, fibrosing mediastinitis, chronic renal failure |
Pulmonary Hypertension and Outcome Studies in Cardiac and Noncardiac Surgery
A paucity of appropriately controlled studies involving surgical patients with PH, varied definitions of PH in case reports and case series, reliance on different methodologies (RHC versus echocardiography studies) for evaluation of PH, and inclusion of patients with varied underlying pathophysiology in these studies have hindered the publication of firm recommendations by clinicians and scientists [19].
While some differences in outcomes have been reported in the literature, PH clearly represents an important risk factor for perioperative morbidity and mortality. Most of these studies of PH patients presenting for noncardiac surgery point to an increased rate of postoperative respiratory failure, delayed tracheal extubation, hemodynamic instability, arrhythmia, heart failure, sepsis, renal insufficiency, and longer intensive care unit (ICU) stay [1, 20–23]. Retrospective outcome studies on noncardiac surgery, although not useful guides for predicting outcomes after HTX and LTX, point to the importance of developing appropriate perioperative management strategies in this complex surgical population. In reviewing the literature on PH patients undergoing cardiac surgery, existing data points to increased morbidity and mortality. Deterioration of preexisting right ventricle (RV) failure has been identified as the leading cause of morbidity and death in this group of patients [24–28]. A relatively large cohort study published in 1999 included 2149 patients undergoing coronary bypass grafting. This study identified PH as an independent predictor of mortality (odds ratio 2.1, P = 0.029) [25].
Pulmonary Hypertension and Thoracic Organ Transplantation
RV function is the main focus in patients with preexisting PH undergoing HTX and LTX. In general, RV failure in ICU patients prognosticates higher morbidity and mortality. Low systemic blood pressure, hyponatremia, and increased levels of plasma brain natriuretic peptide, C-reactive protein, and serum creatinine are some negative prognostic factors for acute right heart failure in PH patients [29, 30]. Myocardial ischemia, endothelial dysfunction induced by endotoxemia, and pro-inflammatory cytokines such as tumor necrosis factor α have been implicated in the pathogenesis of RV dysfunction [31–33]. Studies investigating therapeutic approaches to isolated RV failure are scarce and mostly non-randomized. Of note, Haddad et al. reviewed the literature on RV failure in detail [34, 35].
Pulmonary Hypertension and Outcomes After Heart Transplantation
Although clinicians and scientists agree that preexisting PH prior to HTX portends an increased rate of morbidity and mortality, results of outcome studies are far from consistent and at times conflicting [36–46]. Tenderich et al. retrospectively assessed the long-term survival of 400 consecutive PH patients presenting for orthotopic HTX (OHTX). Enrolled patients were followed over 3.5 years. The authors concluded that post-cardiac transplantation 30-day mortality and cumulative 1- and 5-year survival rates were not adversely affected in PH patients (PVR ≥ 5 WU, TPG > 15 mmHg) compared to the control group [47].
Chang et al. conducted a single center cohort study on 172 HTX recipients (41.3 % of which were defined as having PH). Enrolled patients were followed for up to 15.1 years. The study concluded that mild to moderate preoperative PH was associated with the development of early but not late posttransplantation PH. Although posttransplantation PH was not found to be consistently associated with increased mortality, a potentially worse prognosis was suggested. However, the other conclusion derived from this study indicated that greater than 50 % of first year mortality was attributable to cellular rejection rather than graft failure due to PH [28].
Klotz et al. conducted a prospective study of 217 OHTX recipients (168 patients with normal pulmonary pressure and 49 patients with reversible PH) over 10 years. All PH patients had successful reduction of PVR to ≤2.5 WU and TPG to ≤12 with use of PGI2 or PGE1 prior to HTX. In their study, HTX recipients with reversible PH were found to have significantly higher posttransplantation PAP compared to recipients without PH. These patients were also found to have significantly higher incidences of right heart failure [48].
To evaluate the impact of pretransplant PH, Vakil et al. conducted a large, multicenter cohort study utilizing data derived from 26,649 HTX recipients between 1987 and 2012 (UNOS database). The results demonstrated that the presence of pretransplant PH was a modest but significant predictor of early but not long-term posttransplant mortality. The study also concluded that the increase in adverse events seen in posttransplant patients with preexisting PH occurs, regardless of severity of the disease assessed by PVR, TPG, and MPAP. Additionally, the study demonstrated that institution of mechanical circulatory support as a bridge to transplantation diminishes the incidence and severity of pretransplant PH [4].
Management of Pulmonary Hypertension Before OHTX
Transplant centers have adopted variable cutoff values for PVR and TPG for placing PH patients presenting with end-stage heart failure on transplant lists, and the strategy involved in the process appears to be extremely fluid. According to guidelines issued by the International Society of Heart And Lung Transplantation, the presence of severe pretransplant hypertension, defined as PVR > 5 WU or TPG > 16 mmHg, is associated with early graft dysfunction, increased 30-day mortality, and is considered a relative contraindication for HTX [42, 49]. That being said, no absolute cutoff values for PAP, TPG, or PVR to declare a candidate unsuitable for OHTX have been reported in the literature [45, 50, 51].
RHC in conjunction with pulmonary vasodilator response testing is considered an integrated part of preoperative assessment for OHTX and lung transplant (LTX) candidates. Evidently, less than 10 % of PH patients are considered responsive to acute pulmonary vasodilator testing conducted during RHC. The use of pulmonary vasodilators in “nonresponders” is not recommended clinically and perioperatively [52]. It is noteworthy that the phenomenon of vasoreactivity has not been defined quantitatively and transplant centers have proposed different values as reactive. Acute vasodilator response tests are considered by some experts to be positive when there is a 20 % decline in PVR in response to administration of a pulmonary vasodilator without a reduction of cardiac output [53, 54].
Left ventricular assist device (LVAD) implantation has been used to decrease PVR prior to HTX. Implantation is performed only if raised PVR in an OHTX candidate demonstrates a positive response to continuous infusion of milrinone along with up-titration of pulmonary vasodilator therapy, documented by serial RHC. A number of HTX candidates with severe class 2 WHO PH have benefited from prior implantation of pulsatile or axial-flow ventricular assist devices, leading to successful OHTX with reduced incidence of right heart failure and desirable postoperative PVR [55]. Conversely, RV ischemia and dysfunction is a frequently encountered problem after LVAD implantation. This phenomenon is manifested following separation from cardiopulmonary bypass (CPB) and may require placement of a right ventricular assist device (RVAD) [56, 57]. It has been postulated that the high dP/dT ratio of pulsatile RVAD potentially leads to detrimental injuries of the pulmonary microcirculation, causing a further increase in PVR and elevation of PAP. But theoretically, continuous flow micro-pump modeled RVAD can potentially improve systemic hemodynamics without negatively impacting PVR [58].
Lung and Heart–Lung Transplantation for Pulmonary Hypertension Patients
Currently, the majority of PH patients presenting for LTX undergo double LTX [59]. Single LTX patients have been observed to experience a more complicated postoperative course than their peers receiving double LTX. This is due to ventilation-perfusion mismatch inherent to single LTX surgery [60]. Fewer postoperative deaths [61] and a better survival trend [59] have been noted in double LTX versus single LTX recipients with PH.
PVR declines to normal ranges within 24 h post transplantation (single and double lung), but it takes several weeks for RV dysfunction to resolve. RV hypo-contractility, despite normal PVR, is an important factor contributing to hemodynamic instability and mortality in the immediate postoperative period [62]. Heart–lung transplantation is advocated by some transplant centers for end stage lung disease patients presenting with severe RV dysfunction. However, heart–lung transplantation has not been proven to be superior to double LTX in PH patients, except in patients with Eisenmenger’s syndrome with ventricular septal defect [63]. Additionally, heart–lung grafts are scarce and heart–lung transplantation surgery is a technically complex procedure with longer CPB time. Moreover, postoperative heart–lung recipients may experience cardiac allograft dysfunction, complicating an already difficult postoperative course.
RV systolic function is a product of preload, contractility, afterload, and ventricular interdependence. Ventricular interdependence is a physiological phenomenon that explains how size, shape, and mechanical properties of one ventricle directly impact the other ventricle. Systolic ventricular interdependence is mostly brought about via function of the interventricular septum, whereas for diastolic ventricular interdependence, the pericardium also plays a crucial role. Ventricular interdependence plays a significant role in the pathophysiology of acute RV failure [64]. Ventricular interdependence in RV failure is demonstrated by decreased LV cavity size and impaired LV filling (Fig. 15.2). Post LTX, following resolution of impedance to RV output, LV size and function will eventually normalize. But in the immediate postoperative period, LV diastolic dysfunction will continue to hamper cardiac output and potentially cause donor allograft pulmonary venous engorgement, which in turn may result in pulmonary edema, complicating the postoperative course.
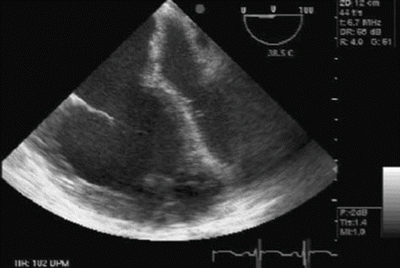

Fig. 15.2
Dilated RV, underfilled left ventricle (LV) and shift of interventricular and interatrial septum to the left indicating acute pulmonary hypertensive crisis in a patient undergoing pulmonary transplantation
Anesthetic Considerations
LTX candidates with PH are considered critically ill and require special anesthesia management plans for their perioperative safety (Table 15.3) [65]. Once the suitability of a donor-recipient pair is concluded, to reduce allograft ischemic time, placement of invasive monitoring and induction of anesthesia should be carried out within a reasonable time frame. The involvement of skilled anesthesiologists specializing in invasive hemodynamic monitoring and airway instrumentation with comprehensive knowledge of the pathophysiology of PH is crucial for the care PH patients undergoing HTX and LTX.
Table 15.3
Safety precautions to avoid pulmonary hypertensive crisis and circulatory collapse during induction of anesthesia
• Continue preoperative pulmonary vasodilators and inotropes (milrinone, prostaglandins) |
• Judicious use of anxiolytics as premedication |
• Position patient in head-up position for comfort with breathing |
• Arterial line placement before induction and continuous blood pressure monitoring during induction |
• Consider central venous catheter and Swan–Ganz catheter placement before induction as appropriate |
• Surgeon and perfusion team members in the operating room during induction |
• Consider preinduction groin exposure and ECMO cannulation in severe compromised patients with suprasystemic pulmonary hypertension |
• Continue pulmonary vasodilators and milrinone through induction and consider starting epinephrine before induction of anesthesia |
• Preoxygenation and hyperventilation after paralysis to avoid hypoxemia and hypercarbia |
• Maintain systemic blood pressure and avoid hypotension (avoid propofol) |
• Smooth and rapid intubation by experienced team member, with single lumen tube if necessary to avoid hypoxemia/hypercarbia |
• Avoid Trendelenberg position for central line placement (venous pressures are high so this position usually with no advantage) |
• TEE probe placement immediately after intubation to evaluate right heart and optimize hemodynamics (preload, afterload, and contractility) |
• Nitric oxide available in the operating room and started immediately after intubation |
Preoperative Considerations
PH-specific medications should be continued preoperatively, since withdrawal can precipitate a rebound and hence PH crisis. Extreme care also needs to be taken to avoid exacerbation of PH secondary to sympathetic stimulation caused by anxiety and pain. Anxiolytics and sedatives (benzodiazepines and narcotics) administered during placement of thoracic epidural catheter and invasive monitoring devices, if used judicially, have minimal impact on systemic vascular resistance (SVR) and PVR.
Another dilemma the transplant team faces is transportation of transplant candidates on high ventilatory support to the operating room. Transport ventilators and bi-level positive airway pressure devices are often inadequate to provide necessary support. These patients may not withstand hypoxia- and hypercarbia-induced elevation of PVR and are prone to develop circulatory failure due to acute RV decompensation. Commonly, patients with severe RV failure and near systemic PH may require establishment of extracorporeal membrane oxygenation (ECMO) in the ICU prior to transport to the operating room or before induction of anesthesia in the operating room.
Induction of Anesthesia
Perturbation of hemodynamics during induction should be mitigated by all means possible. Avoidance of PH crisis and systemic hypotension are the two major goals for anesthesia management in PH patients. PAP is maintained preferably within the 15 % range of preoperative values to avoid acute RV decompensation [66]. Insertion of a pulmonary artery catheter (PAC) before induction of anesthesia to monitor PAP and cardiac index (CI) can be useful. Central venous access inserted before induction also ensures reliable delivery of inotropes and vasopressors if needed. However, insertion of PAC and central access in a patient who cannot lie supine because of their compromised cardiorespiratory status can be challenging. Administration of sedation during the procedure can further exaggerate hypoventilation.
Most anesthetic agents administered in induction doses potentially have the ability to depress cardiac contractility and SVR precipitously. Patients with fixed PH are prone to circulatory collapse in the face of abrupt sympatholysis associated with induction of anesthesia [67, 68]. Maintenance of systemic blood pressure and SVR plays a major role in the prevention and management of RV dysfunction. Stages of right heart failure in patients with pulmonary hypertension are explained in Fig. 15.3. Slow progression of PVR in long-standing PH would allow the RV to adapt and hypertrophy; therefore, PH patients theoretically better tolerate abrupt increases in PVR. As RV hypertrophies and RV systolic pressure approaches aortic root systolic pressure, RV perfusion during systole is decreased or completely ceased. Therefore, higher systemic pressure will be required to maintain adequate RV perfusion. As PH progresses and the RV fails, the rise of RV end diastolic pressure further compromises RV perfusion, which is limited to diastole. Increased oxygen consumption from any stress results in RV ischemia and failure [69]. Therefore, systemic hypotension needs to be preempted and treated by all means possible during induction and maintenance [31, 70]. In order to prevent circulatory failure and emergency institution of CPB following induction of anesthesia, establishment of ECMO prior to induction of anesthesia has been advocated by some authors [71].
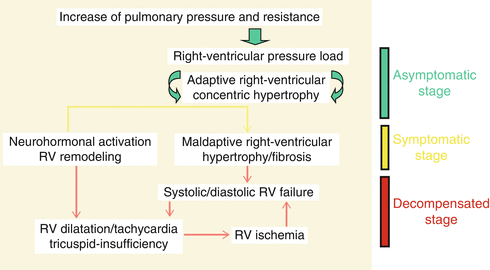

Fig. 15.3
Pathophysiology of RV dysfunction in pulmonary hypertension (Adapted from Gille et al. [173].)
Airway Management
Patients presenting for organ transplantation may not be fasting due to the time constraints attributable to the logistics of transplantation. If a transplant candidate is judged to have a full stomach due to inadequate preoperative fasting or decreased gastric emptying secondary to gastroparesis, following adequate preoxygenation with 100 % O2, modified rapid-sequence induction (ventilation with cricoid pressure) is carried out. Otherwise, effective mask ventilation to avoid hypoxia and hypercarbia prior to instrumentation for intubation is required. Smooth induction and tracheal intubation while avoiding hypoxia and hypercarbia is not only desired but also essential. Also, with initiation of positive pressure ventilation, intrathoracic pressure should be monitored closely to prevent an unwanted increase in plateau pressure. Airway instrumentation following induction of anesthesia and awake fiber-optic intubation in patients deemed to be difficult intubation cases must be carried out by the most skilled anesthesiologist present to prevent undue sympathetic stimulation, hypoxia, and hypercarbia. Dexmedetomidine is an appropriate agent to facilitate awake fiber-optic intubation. As an agent that can provide sedation and analgesia without untoward depression of spontaneous respiration, dexmedetomidine has been successfully used to allay undue anxiety and alleviate pain.
Anesthesia Drugs and Pulmonary Hypertension
Etomidate has been recommended as the induction agent of choice for PH patients presenting for OHTX and LHTX [72]. Etomidate allows stable induction of anesthesia due to its minimal impact on SVR and myocardial contractility [73, 74]. The other commonly used induction agent propofol is not considered an appropriate choice for PH patients [73]. Propofol , when used in induction doses, can cause RV hypoperfusion secondary to a precipitous drop of SVR. Thiopental reduces SVR and RV contractility without impacting PVR [75], making it unsuitable for induction of anesthesia in PH patients. Although ketamine has been found to increase PVR in adults [76], it does not appear to increase PVR when used in conjunction with pulmonary vasodilators [18]. Morray et al. studied the hemodynamic effects of ketamine in children with congenital heart disease undergoing cardiac catheterization and concluded that ketamine neither altered the patients’ clinical status nor impacted the information obtained by cardiac catheterization [77]. Williams et al. indicated that ketamine could be used as an induction agent in PH children undergoing congenital heart defect repair. Ketamine was demonstrated to not increase PVR in PH children undergoing sevoflurane anesthesia and spontaneous ventilation [78].
As part of a balanced anesthetic technique, narcotics and inhalational agents (≤1 MAC), if used with diligence, do not significantly affect hemodynamics. Fentanyl and sufentanil were also found to minimally impact PVR [79]. Inhalational anesthetic agents (isoflurane, desflurane) inhibit hypoxic pulmonary vasoconstriction and hence can further exacerbate hypoxemia. They also cause a dose-dependent decrease in SVR and myocardial contractility and impair RV-pulmonary artery coupling, which can be detrimental in the face of RV dysfunction [80, 81].
Intraoperative Monitoring
In addition to standard American Society of Anesthesiologists monitors (electrocardiography, pulse oximetry, temperature, exhaled end tidal carbon dioxide), indwelling arterial catheter (radial and femoral), PAC, and transesophageal echocardiography (TEE) are required monitoring devices during LTX and HTX surgeries (Fig. 15.4). In addition to direct measurements of PAP and PCWP, PAC is capable of measuring and calculating PVR, RV stroke work index, CI, ejection fraction, and mixed venous oxygenation (SvO2) [82, 83].
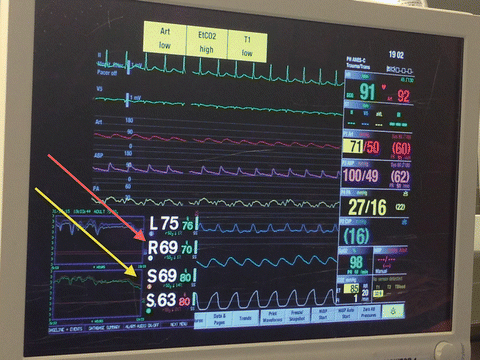

Fig. 15.4
Typical standards of monitoring in patients undergoing lung transplantation [EKG, invasive BP (Femoral-Purple, Radial-Red), pulmonary arterial pressure (yellow), central venous pressure (blue), pulse oximetry, capnography, temperature, and cerebral (red arrow)/leg (yellow arrow) near infrared spectroscopic oximetry (arrows)]
Alternatively, continuous cardiac output (CCO)/mixed venous oxygen saturation (SvO2) monitoring PACs can be used instead of regular PACs to provide clinicians with instantaneous information, facilitating pharmacological, fluid, and ventilatory interventions in a timely fashion. SvO2, being an indicator of oxygen-carrying capacity, is also a useful guide in guiding the transfusion of red blood cells (Fig. 15.5). Transfusion of blood products, if not warranted for tissue perfusion or coagulation, has been proven to increase morbidity without additional benefit. Specifically, in the cardiac surgery setting, RBC transfusion has been associated with infection, ischemia, and early and late mortality [84]. That being said, although an optimal hemoglobin concentration in heart failure has not yet been established, both systolic and diastolic heart failure patients with anemia have been found to have higher mortality rates [85].
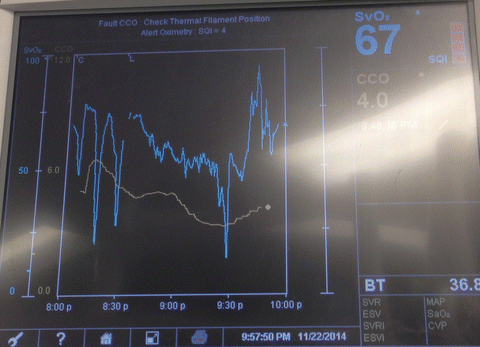

Fig. 15.5
The use of oximetric-continuous cardiac output Swan-Ganz catheters display useful information (Continuous cardiac output (CCO) and mixed venous oxygen saturation (SVO2) in patients undergoing lung transplantation
Cardiac output measured using the PAC thermodilution method is deemed to be inaccurate in the face of significant TR, anatomical shunts, and tachyarrhythmia [83]. Therefore, in patients with significant TR and anatomical shunts, TEE will provide a more accurate estimation of cardiac output than PAC. Although PAC has not been found to impact outcome [86], especially when used in conjunction with TEE , PAC is a useful tool to assess the efficacy of treatment strategies. It also facilitates the titration of vasoactive agents to desirable end points.
Unlike TEE , PAC can also be continued for hemodynamic evaluation and management postoperatively in the ICU after transplantation. Any persistent rise in PAP or central venous pressure (CVP) associated with a fall in CI postoperatively should be investigated by TEE for specific diagnosis (pericardial tamponade, RV dysfunction).
Maintenance of Hemodynamics, Ventilation, and Fluid Management
Patients undergoing HTX and LTX experience dramatic fluid shifts and changes in hemodynamics and ventilation during surgery. Avoiding all aggravating factors possible and attenuating the effects of stress, pain, sympathetic stimulation, and inflammation induced by surgery are essential to avoid PH crisis. PH crisis is defined as the acute onset RV failure secondary to abrupt increase of PVR (Table 15.4) [2, 87, 88]. Atrial fibrillation or third-degree atrioventricular block with its resultant increased filling pressure and decreased cardiac output are poorly tolerated in patients with acute RV dysfunction [89]. These rhythm abnormalities are a common occurrence in patients with baseline gaseous and metabolic abnormalities and can also be precipitated by the manipulation of the heart. Therefore, loss of sinus rhythm in the setting of acute RV dysfunction must be treated aggressively using synchronized cardioversion or pacing as needed.
Table 15.4
Factors that adversely affect pulmonary vascular tone and hypertension
Inadequate depth of anesthesia |
Inadequate pain management |
Administration of vasoactive medications |
Excessive fluid administration |
Lung volumes (excessive PEEP, inadequate ventilation with atelectasis) |
Increased airway pressure |
Hypoxemia |
Hypercarbia and respiratory acidosis |
Metabolic acidosis (inadequate tissue oxygen delivery from multiple causes; hypovolemia, vasopressors, hypoxemia, hypotension, cardiac manipulations, reduced cardiac output) |
Cardiac arrhythmias (atrial fibrillation, supraventricular tachycardia) |
Hypothermia |
Since there is an immense potential for fluid shifts in HTX and LTX surgeries, RV preload should be monitored closely and intravenous boluses and infusions must be administered judiciously to prevent acute RV distention. A failing, hypertrophied RV will neither tolerate hypervolemia nor hypovolemia; therefore, goal-oriented fluid therapy in transplantation is of pivotal importance. Each case needs a carefully tailored fluid administration strategy, best dictated by real-time TEE findings. In addition to RV distention, increasing severity of TR and leftward interventricular septal shift should give important clues to anesthesiologists regarding fluid status. Another important TEE-derived indicator, tricuspid annular plane systolic excursion (TAPSE) , is an important diagnostic tool in the setting of acute RV dysfunction [90–92]. TAPSE , which is quantified using M-mode echocardiography in four-chamber view or transgastric view, reflects longitudinal systolic excursion of tricuspid annulus towards apex and is used for quantitative assessment of RV systolic function (Fig. 15.6) [93]. Also, rise of RA pressure in the presence of patent foramen ovale would exacerbate hypoxemia, further deteriorating an already precarious situation.
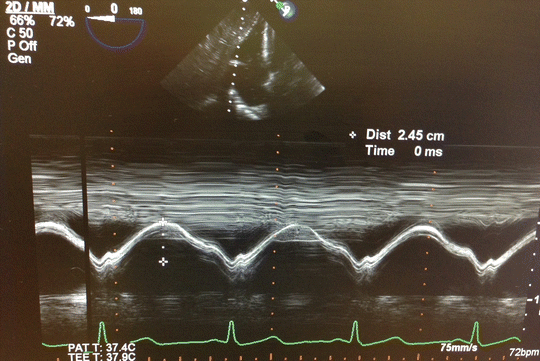

Fig. 15.6
Tricuspid annular plane systolic excursion (TAPSE) measured by M-mode transesophageal echocardiography transgastric view is used to evaluate RV function. TAPSE 24.5 mm indicates good RV function. TAPSE less than 10 mm indicates severe RV dysfunction
In the immediate postoperative period when real-time TEE assessment of RV contractility and filling status is no longer available, intraoperative, PAC information, which corresponds to TEE findings indicative of adequate RV filling, can potentially serve as a good guide for fluid therapy. The ultimate goal is to provide adequate RV preload without causing undue distention and/or interventricular septal shift. Another strategy that could be employed in the ICU setting is administration of 250 ml boluses of fluid while following CVP, PCWP, and systemic arterial pressures simultaneously. A rise in CVP that correlates with an increase in PCWP and mean arterial pressure is a sign that things are moving in right direction. Conversely, if a rise in CVP doses not correspond with positive changes in PCWP, further fluid administration should be halted. Intuitively, a simultaneous decrease in cardiac output and increase in systemic venous pressure manifested by a rise in CVP, which eventually leads to multiorgan failure, should be prevented by all means possible. A failing RV is incapable of generating high pressures in the pulmonary arterial system. Therefore, it is important to note that declining PAP associated with a rise in CVP is an indicator of worsening RV function rather than decreased PVR. This can be confirmed by demonstration of a dilated hypocontractile RV on real-time echocardiography.
Optimal respiratory management for PH patients would include a strategy to ensure adequate oxygenation and ventilation without an undue increase in intrathoracic pressure [94, 95]. Induction of anesthesia and institution of mechanical ventilation, per se, cause an increase in intrathoracic pressure, resulting in decreased RV preload and cardiac output [66]. Once mechanical ventilation is established, a “U”-shaped relationship between lung volumes and PVR can be appreciated, with lung volumes close to functional residual capacity, providing the most favorable pulmonary and hemodynamic profile [18]. Intuitively, higher intrathoracic pressures brought about by higher tidal volume and high positive end expiratory pressures (PEEP) may adversely affect RV afterload, increase TR and RA pressures, and further deteriorate RV function. Also, higher PEEP values could cause compression of the pulmonary vasculature in well-ventilated areas of the lungs and divert blood to poorly ventilated areas, resulting in pulmonary shunting and hypoxemia. At times, balancing adequate oxygenation and ventilation along with avoidance of high airway pressure could be a tasking challenge in patients presenting with poor lung compliance, requiring sophisticated ICU ventilator and special ventilator strategies.
A ventilation strategy with a smaller tidal volume (6–8 ml/kg of ideal body weight) and higher respiratory rate (16–20) to target acceptable pH (between 7.25 and 7.4) is usually implemented [18, 66, 96]. Although hyperventilation with increasing respiratory rate can potentially decrease PVR by attenuating acidosis-induced pulmonary vasoconstriction, it should be implemented cautiously to avoid dynamic hyperinflation. Lung protective respiratory management strategies (utilizing low tidal volume 6–8 ml/kg and airway pressure <30 cmH2O) are widely used in ICUs as an integrated part of postoperative care of patients with acute lung injury. Moreover, low tidal volume strategy has been proven to improve endothelial dysfunction via reduced cytokine production [97]. Following LTX, mechanical ventilation with a lung-protective strategy avoiding alveolar hyperinflation is recommended. Administration of optimal PEEP with lowest FiO2 possible to prevent oxygen toxicity while ensuring SaO2 > 90 % are important ventilatory considerations for preventing allograft dysfunction (Fig. 15.7). Appropriate postoperative ventilatory management plays a crucial role in protecting donor grafts in LTX patients and also in preventing undue increases in PVR in both HTX and LTX recipients.
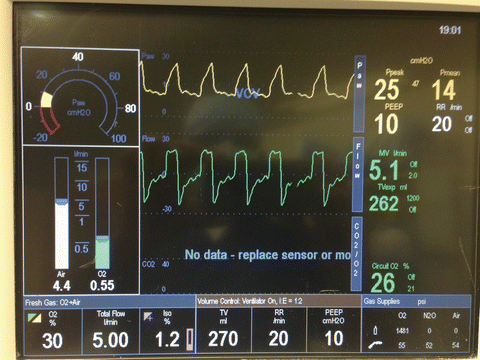

Fig. 15.7
Lung protective ventilator settings after lung transplantation (Low FIO2, low tidal volume, high respiratory rate, moderate PEEP, and airway pressure less than 30 mmHg)
Circulatory Support for Transplantation
While HTX is always performed using CPB, LTX can be conducted in some patients with PH without circulatory support. Preoperative oxygen requirement at rest (liters/min), increasing MPAP, dilated RV, severe tricuspid regurgitation, and severely depressed RV function were found to predict the need for CPB (unpublished observation). Avoidance of CPB reduced the days of postoperative ventilation and the need for tracheostomy in our PH patient cohort. Therefore, every effort is made to avoid CPB in patients with mild and moderate PH while severe PH patients will always require CPB for the procedure.
In bilateral sequential single LTX, further deterioration of oxygenation and ventilation during single lung ventilation may ensue, culminating in an acute rise of PVR, precipitating RV decompensation and circulatory collapse. This dreaded complication could be partly mitigated by dissecting the lung with inferior V-Q mismatch first. Additionally, inhaled pulmonary vasodilators and ino-vasotropic support may allow lung dissection without resorting to CPB. If ventilatory and pharmacological interventions are deemed futile, extracorporeal circulation (CPB or ECMO) will be necessary. ECMO can very well be extended to the postoperative period if allograft dysfunction results in intractable hypoxemia [68, 71, 98–100].
Clamping of the pulmonary artery is considered to be a perilous stage of LTX surgery requiring special preparation. During pulmonary artery clamping, acute rises in PAP may result in near fatal RV dilatation, necessitating emergency establishment of CPB. That being said, it is abundantly evident that avoidance of CPB mitigates deleterious inflammatory effects such as coagulopathy and allograft dysfunction [101]. To avoid CPB, anesthesiologists need to preemptively implement ino-chronotropic, vasoconstrictor, and pulmonary vasodilatory support. Also, temporary clamping of the respective pulmonary artery prior to definite ligation would allow the surgeon and the anesthesiologist to evaluate the feasibility of transplantation without resorting to CPB, avoiding hazardous emergency cannulation and “crash bypass.”
At times, surgical manipulations such as retraction required for exposure needed for left lung-atrial anastomoses could result in severe hemodynamic compromise, mandating institution of CPB. Institution of CPB in a timely fashion is advisable to avoid surgical complications secondary to emergency cannulation required for CPB.
Also, CPB may not be required for the second lung graft and hence terminated to decrease the duration of “on pump” time. Another critical stage in LTX surgery is the unclamping of pulmonary vessels. Profound systemic hypotension following completion of allograft anastomoses and unclamping of the pulmonary vasculature must be preempted and treated aggressively with up-titration of inotropes and vasoconstrictors. Additionally, fluid administration should be monitored closely to provide adequate RV preload while avoiding RV distention. One of the etiologies for posttransplantation pulmonary edema in donor graft is overzealous fluid therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





