Osmolarity refers to the concentration of osmotically active particles in a solution expressed in milliosmoles per liter (mOsm/L)
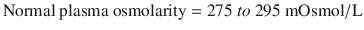
Plasma osmolarity is calculated based on the following osmotically active particles in plasma =
![$$ 2\left[\mathrm{Na}+\right]+\mathrm{Glucose}/18+\mathrm{BUN}/2.8 $$](/wp-content/uploads/2017/12/A340077_1_En_15_Chapter_Equc.gif)
![$$ 2\left[\mathrm{Na}+\right]+\mathrm{Glucose}/18+\mathrm{BUN}/2.8 $$](/wp-content/uploads/2017/12/A340077_1_En_15_Chapter_Equc.gif)
15.1.1 Osmolarity Versus Tonicity
Both osmolarity and tonicity compare the solute concentrations of 2 solutions separated by a membrane.
Osmolarity takes into account the total concentration of penetrating solutes and non-penetrating solutes, while tonicity takes into account the total concentration of only non-penetrating solutes.
15.2 Crystalloids
Crystalloids are intravenous fluids containing electrolytes and/or dextrose dissolved in water. ◘ Table 15.1 lists the composition of commonly used intravenous crystalloid solutions.
Table 15.1
Composition of commonly used crystalloid solutions
Solution | mOsm/L | Tonicity | pH | Na+a | Cl−a | K+a | Ca2a | Mg2a | Glucose g/L | Lactatea |  a a | Gluconatea | Acetatea |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Lactated Ringer’s (LR)b | 273 | Iso | 6.5 | 130 | 109 | 4 | 3 | – | – | 28 | – | – | – |
Plasmalyte A | 294 | Iso | 7.4 | 140 | 98 | 5 | – | 3 | – | – | – | 23 | 27 |
Plasmalyte – 148 | 294 | Iso | 5.5 | 140 | 98 | 5 | – | 3 | – | – | – | 23 | 27 |
Normal Saline (NS) | 308 | Iso | 5.0 | 154 | 154 | – | – | – | – | – | – | – | – |
5% dextrose (D5) in water | 253 | Hypo | – | – | – | – | – | – | 50 | – | – | – | – |
D5 ¼ NS | 355 | Iso | 4.0 | 38.5 | 38.5 | – | – | – | 50 | – | – | – | – |
D5 ½ NS | 406 | Hyper | 4.3 | 77 | 77 | – | – | – | 50 | – | – | – | – |
D5 NS | 586 | Hyper | 4.0 | 154 | 154 | – | – | – | 50 | – | – | – | – |
D5 LR | 525 | Hyper | 5.0 | 130 | 109 | 4 | 2.7 | – | 50 | 28 | – | – | – |
½ NS | 154 | Hypo | 77 | 77 | – | – | – | – | – | – | – | – | |
3% Saline (S) | 1027 | Hyper | 5.0 | 513 | 513 | – | – | – | – | – | – | – | – |
5% S | 1711 | Hyper | 5.0 | 856 | 856 | – | – | – | – | – | – | – | – |
7.5% NaHCO3 | 1786 | Hyper | – | 893 | – | – | – | – | – | – | 893 | 23 | 27 |
Normasol | 280 | Iso | 7.4 | 140 | 98 | 5 | – | – | – | – | – | 23 | 27 |
15.2.1 NaCl 0.9%
Infusion of 0.9% NaCl is accompanied by a hyperchloremic metabolic acidosis, especially if large volumes are administered. Its effects on the kidneys include renal vasoconstriction, reduced glomerular filtration rate (GFR), and a reduction in renal cortical perfusion. Observational trials showed that the use of isotonic normal saline could increase the incidence of acute kidney injury (AKI) and the requirement for renal replacement therapy, likely through a reduction in renal perfusion. In a recent randomized trial of critically ill postoperative (non trauma) patients, the use of buffered crystalloid solutions did not reduce the risk of acute kidney injury.
15.2.2 NaCl 3%
The use of hypertonic NaCl is limited to cases of increased intracranial pressure to reduce cerebral edema as well as for the treatment of hypoosmolar hyponatremia. Its use as a volume expander has been considered in prehospital trauma with no demonstrable improvement in patient outcomes.
15.2.3 Balanced Crystalloid Solutions
Balanced crystalloid solutions including plasmalyte (isotonic) and Ringer’s lactate, gluconate, and acetate (mildly hypotonic) have a lower sodium and chloride concentration as compared to isotonic NaCl. The reduced anion content is compensated for by the addition of buffers such as lactate, gluconate, and acetate. Following administration, the buffer is metabolized to 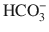 . Balanced crystalloid solutions avoid the hyperchloremic metabolic acidosis caused by isotonic NaCl. In addition, they reduce antidiuretic hormone (ADH) secretion, thereby reducing the typical salt and water retention that occurs with isotonic NaCl administration, and allow diuresis in response to intravascular fluid expansion.
. Balanced crystalloid solutions avoid the hyperchloremic metabolic acidosis caused by isotonic NaCl. In addition, they reduce antidiuretic hormone (ADH) secretion, thereby reducing the typical salt and water retention that occurs with isotonic NaCl administration, and allow diuresis in response to intravascular fluid expansion.
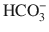 . Balanced crystalloid solutions avoid the hyperchloremic metabolic acidosis caused by isotonic NaCl. In addition, they reduce antidiuretic hormone (ADH) secretion, thereby reducing the typical salt and water retention that occurs with isotonic NaCl administration, and allow diuresis in response to intravascular fluid expansion.
. Balanced crystalloid solutions avoid the hyperchloremic metabolic acidosis caused by isotonic NaCl. In addition, they reduce antidiuretic hormone (ADH) secretion, thereby reducing the typical salt and water retention that occurs with isotonic NaCl administration, and allow diuresis in response to intravascular fluid expansion.15.2.4 Dextrose Solutions
Dextrose solutions are typically used for acute management of hypoglycemia (D50) and for maintenance of patients’ nutritional requirements as part of parenteral nutrition. Dextrose solutions are not suitable for intravascular fluid expansion, since water is able to move across fluid compartments and is not confined to the intravascular space. Glucose solutions can also be used in conjunction with insulin for management of hyperkalemia (by shifting K+ intracellular) as well as to reduce the occurrence of hypoglycemia in diabetic patients receiving insulin infusions.
15.2.5 Crystalloids To Be Avoided in Certain Conditions and Disease States
Acute and chronic kidney disease: Avoid large volumes of 0.9% NaCl.
Hyperparathyroidism: Avoid Ca2+ containing solutions including Ringer’s lactate
Blood product transfusion: Avoid Ca2+ containing solutions to prevent clotting caused by interaction of Ca2+ with citrate. The small amounts of Ca2+ in Ringer’s lactate make this a rare and hypothetical occurrence.
Traumatic brain injury: Avoid hypotonic solutions for fear of worsening cerebral edema; avoid glucose containing solutions for fear of worsening of neurologic outcome.
Liver Disease: Avoid hypotonic solutions that may exacerbate ascites/interstitial edema due to a reduced oncotic pressure.
Hyperglycemia: Avoid glucose containing solutions to prevent worsening hyperglycemia
Increased Intracranial Pressure: Avoid hypotonic solutions for fear of worsening cerebral edema.
15.3 Colloids
Colloids are very small, finely divided particles that remain dispersed in a liquid for a long time due to their small size (usually less than 1 mm) and electron charge. While these particles are too small to be seen by the optical microscope, they are too big to pass through a semipermeable membrane. These particles have negligible settling velocity because of their small mass and have a low gravitational force compared to surface frictional forces. Colloids can be classified into natural and synthetic colloids. Albumin is the typical example of natural colloids, while synthetic colloids include various hydroxyethyl starches, dextran, and gelatins.
15.3.1 Albumin
Human serum albumin is a 66.5-kDa, negatively charged, and elliptically shaped protein that contributes up to 80% of the intravascular oncotic pressure and serves as a transport protein in blood. Albumin 5–25% is a sterile liquid solution that contains variable concentrations of albumin (5–25%) in normal saline. Albumin is derived from large pools of human plasma, manufactured by cold ethanol fractionation followed by ultra- and diafiltration. The manufacturing also includes final container pasteurization and additional bulk pasteurization at 60 ± 0.5 °C for 10–11 h. The combination of fractionation and pasteurization virtually eliminates the risk of potential viral transmission.
The effective oncotic pressure of the 5% albumin solution approaches that of human plasma (around 25 mmHg) while that of the 25% solution is approximately four to five times that of human plasma.
Albumin traverses the intravascular space, a phenomenon known as “transcapillary filtration,” into the interstitial space via both passive filtration at areas with large gaps in the endothelium and active filtration via the receptor albondin. Albondin is an avid receptor for the transport of albumin into the interstitial space, but is lacking in certain tissue compartments, such as brain, resulting in low albumin levels in the cerebrospinal fluid. In experimental animal models, however, albumin has been shown to interact with the endothelial glycocalyx, thereby preserving the integrity of the endothelial surface layer and more effectively limiting fluid extravasation compared to crystalloids and other synthetic colloids.
While most studies report that albumin is safe but not significantly better than saline (0.9% NaCl) in reducing mortality, albumin has been shown to be beneficial for the following patient populations:
Hypoalbuminemia and multiorgan dysfunction: Dubois et al. used intravenous albumin to correct hypoalbuminemia, and used the Sequential Organ Failure Assessment (SOFA) score to evaluate for changes in organ dysfunction. They concluded that the administration of albumin to critically ill hypoalbuminemic patients showed improved organ function especially in the central nervous system, the cardiovascular system, and the pulmonary system.
Sepsis: A meta-analysis reported that the use of albumin-containing solutions for the resuscitation of patients with sepsis was associated with lower mortality.
Spontaneous bacterial peritonitis complicating cirrhosis.
Children with severe malaria.
On the other hand, several studies reported an increase in mortality with the use of albumin in patients with traumatic brain injury. While the exact mechanism is not well understood, a disruption in the blood brain barrier with leakage of albumin into the brain parenchyma causing rebound intracranial hypertension has been suggested.
15.3.2 Hydroxyethyl Starch
Hydroxyethyl starch (HES) is a synthetic colloid solution in which the molecular mass of at least 80% of polymers ranges between 10 thousand to 2 million daltons (Da). Larger molecules are degraded enzymatically by amylase. HES is stored in the reticuloendothelial system for several hours and is believed to be renally excreted. HES is mixed in a salt solution so that its salt concentration is similar to that in blood. HES has become one of the most frequently used colloidal plasma expanders worldwide, mainly due to its lower cost compared to albumin. Most HES preparations have oncotic pressures around 30 mmHg, (hetastarch: 450/0.7, Voluven®: 130/0.4) with some preparations having much higher oncotic pressures of 55 mmHg (pentastarch: 260/0.5).
HES is derived from amylopectin, a polysaccharide from maize, and is similar to glycogen. HES is prepared by hydrolysis, in vivo, by serum alpha-amylase and amylopectin hydroxyethylation. HES is excreted by the kidneys and the rate of decomposition of starch is based on the molar substitution and C2/C6 ratio. The higher the molar substitution and C2/C6 ratios, the slower the decomposition, ultimately leading to plasma accumulation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






