Pericardiocentesis
Richard C. Becker
Pericardiocentesis is a potentially life-saving procedure performed in the critical care setting. It is not carried out with sufficient frequency, however, to allow most physicians to master the procedure. This chapter reviews the indications for emergent and urgent pericardiocentesis, summarizes the pathobiology of pericardial effusions, and provides a step-by-step approach to pericardiocentesis, including management of patients following the procedure.
Indications for Pericardiocentesis
The initial management of patients with either a known or suspected pericardial effusion is largely determined by their overall clinical status. In the absence of hemodynamic instability or suspected purulent bacterial pericarditis, there is no need for emergent or urgent pericardiocentesis. It may, however, be performed for diagnostic purposes. A thorough noninvasive workup should be completed before consideration of an invasive diagnostic procedure [1]. Whenever possible, elective pericardiocentesis should be performed using echocardiographic guidance.
In contrast, the management of hemodynamically compromised patients requires emergent removal of pericardial fluid to restore adequate ventricular filling (preload) and hasten clinical stabilization. The exact method and timing of pericardiocentesis is ultimately dictated by the patient’s overall degree of instability. Patients with hypotension unresponsive to fluid resuscitation and vasopressor support require immediate, often unguided (blind), pericardiocentesis. In this setting, there are no absolute contraindications to the procedure, and it should therefore be performed without delay at the patient’s bedside.
Urgent pericardiocentesis is indicated for patients who are initially hypotensive but respond quickly to aggressive fluid resuscitation. The procedure should be performed within several hours of presentation while careful monitoring and hemodynamic support continue. As in elective circumstances, pericardiocentesis in these patients should be undertaken with appropriate visual guidance, the method of which depends on the physician’s expertise and resources. The modalities used most commonly are echocardiography or computed tomography (CT).
Three additional points must be stressed regarding patients undergoing expedited pericardiocentesis. First, coagulation parameters—prothrombin time, partial thromboplastin time, and platelet count—should be checked and, when possible, quickly normalized prior to the procedure. An anti-Xa level is recommended for patients receiving low molecular weight heparin. Second, many critical care specialists and cardiologists advocate performance of all pericardiocentesis procedures in the catheterization laboratory with concomitant right heart pressure monitoring to document efficacy of the procedure and to exclude a constrictive element of pericardial disease [2]. The authors support this approach; however, excessive delays must be avoided. Finally, efforts to ensure a cooperative and stationary patient during the procedure greatly facilitate the performance, safety, and success of pericardiocentesis.
The clinical presentation of hemodynamically significant pericardial effusions varies widely among patients. A comprehensive understanding requires knowledge of normal pericardial anatomy and physiology.
Anatomy
The pericardium is a membranous structure with two layers separated by a small potential space. The visceral pericardium is closely but loosely adherent to the epicardial surface. It is a monolayer of mesothelial cells and attaches to the epicardium by a loose collection of small blood vessels, lymphatics, and connective tissue. The parietal pericardium is a fibrous structure that defines the outer membrane. Its inner surface is also composed of a monolayer of mesothelial cells. The remainder of the parietal pericardium consists of a dense network of connective tissue that is relatively nondistendible; therefore, it defines the dimensions and shape of the pericardium [3].
Further anatomic definition of the pericardium is derived from multiple attachments of the parietal pericardium in the thorax. Superiorly, the fibrous parietal pericardium attaches to the ascending aorta just below the arch. The inferior portion adheres strongly to the fibrous center of the diaphragm on which it rests. Anteriorly, the outer membrane is anchored to the sternum and costal cartilages by ligaments, as well as by a less-organized collection of connective tissue. The posterior margin of the parietal pericardium abuts the esophagus and pleural sacs; here, the visceral pericardium is absent and the parietal pericardium attaches directly to the epicardium at the borders of the entrance of the inferior and superior vena cavae and pulmonary veins [4]. Beyond providing stability, these multiple attachments also limit the inherent elasticity and distensibility of the pericardium.
This complex anatomic arrangement provides an anchor for the contracting myocardium and results in a small space between the visceral and parietal layers (pericardial space). The pericardial space or sac usually contains a small volume (15 to 50 mL) of clear, serous fluid that is chemically similar to a plasma ultrafiltrate [5, 6]. The mechanism responsible for the production of pericardial fluid is not well understood. A homeostasis usually exists between new production of pericardial fluid and its drainage into the venous circulation via lymphatics.
The major determinant of when and how pericardial effusions come to clinical attention is directly related to the speed at which they develop. Effusions that collect rapidly (over minutes to hours) may cause hemodynamic compromise with volumes of 250 mL or less. These effusions are usually located posteriorly and are often difficult to detect without echocardiography or other imaging modalities such as multislice CT or cardiac magnetic resonance imaging (MRI). In contrast, effusions developing slowly (over days to weeks) allow for hypertrophy and distention (stretch) of the fibrous parietal membrane. Volumes of 2,000 mL or greater may accumulate without significant hemodynamic compromise. These patients may present with symptoms owing to compression of adjacent thoracic structures, such as cough, dyspnea, dysphagia, or early satiety. Three other clinical conditions promote hemodynamic compromise, even in the absence of large pericardial effusions: intravascular hypovolemia, impaired ventricular systolic function, and ventricular hypertrophy with decreased elasticity of the myocardium (diastolic dysfunction).
Procedure
Since the first blind or closed pericardiocentesis performed in 1840 [7], several different approaches have been described [8]. These approaches have varied considerably, particularly in the needle apparatus entry site. Marfan [9] described the subcostal approach in 1911, which then became the standard approach for unguided pericardiocentesis.
The advent of clinically applicable ultrasonography has opened a new chapter in diagnostic and therapeutic approaches to pericardial disease, allowing clinicians to quantitate and localize pericardial effusions quickly and noninvasively [10,11]. Work by Callahan et al. [12,13] at the Mayo Clinic established the efficacy and safety of two-dimensional echocardiography to guide pericardiocentesis. This has resulted in two major trends in clinical practice: First, two-dimensional echocardiography is commonly used to guide pericardiocentesis. Second, approaches other than the traditional subxiphoid method have been investigated owing to the ability to clearly define the anatomy (location and volume) of each patient’s effusion [8, 12, 13]. Typically, a four-chamber view of the heart is obtained by positioning the transducer at the apex. After insertion of the pericardiocentesis needle (described later), appropriate positioning within the pericardial space can be confirmed by injecting 5 mL of agitated saline (contrast). Echocardiography can also be used to reposition the needle safely if fluid return is suboptimal. Standard fluoroscopy can be used to confirm needle and catheter positioning within the pericardial space.
Formulas for quantitating the amount of pericardial fluid by echocardiographic or fluoroscopic means have not been established. As a rule, however, an effusion of moderate size (at least 250 mL) is required for percutaneous pericardiocentesis.
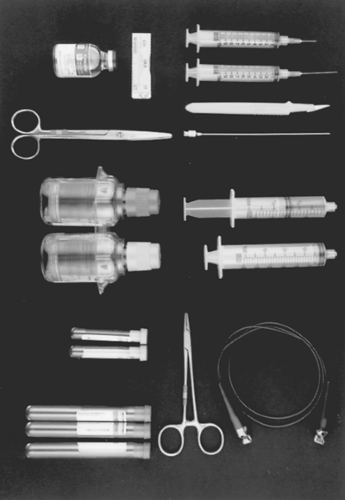
Regardless of whether echocardiography or another guidance method is used, the subxiphoid approach remains the standard of practice. The materials required for bedside pericardiocentesis are listed in Table 7-1 (Fig. 7-1). Table 7-2 (Fig. 7-2) lists the materials required for simultaneous placement of an intrapericardial drainage catheter. The materials are available in prepackaged kits or individually. I do not have a preference; the key to success is immediate availability of the necessary materials.
TABLE 7-1. Materials for Percutaneous Pericardiocentesis | |
|---|---|
|
TABLE 7-2. Materials for Intrapericardial Catheter | ||
|---|---|---|
|
While the patient is being prepared for emergent or urgent pericardiocentesis, it is imperative that aggressive resuscitation measures are undertaken. Two large-bore peripheral intravenous lines should be placed for infusion of isotonic saline or colloid solutions. The use of inotropic agents and other vasoactive drugs (vasopressors) remains controversial [14, 15 and 16], but when fluid resuscitation alone is inadequate, their use should be considered strongly.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree