CHAPTER 29
Percutaneous Sacroplasty
INTRODUCTION
Sacral insufficiency fractures are a common, but often underdiagnosed source of low back pain in the elderly osteoporotic patient. Fractures of the pelvis are a consequence of undue stress onto a weakened bone. Osteoporosis is the most common cause of fractures of the pelvis. Major or minor trauma is another cause; however, spontaneous sacral insufficiency fractures are also common. The incidence of sacral insufficiency fractures comprises of approximately 1% to 2% of pathologic fractures involving the spine and pelvis. However, these fractures are often misdiagnosed and unrecognized. Risks of sacral insufficiency fractures are very similar to that of vertebral compression fractures.
The treatment of sacral insufficiency fractures can either be noninterventional, interventional, or surgical. Unstable fractures, especially with associated cauda equina syndrome, may require closed manipulation or open reduction and internal fixation procedures. Open reduction procedures as compared to percutaneous reduction have increased risks, especially infection.
• Conventionally, treatment in the past has been mainly bed rest, opiate analgesic management, using a walker with partial weight-bearing and early mobilization, and lumbosacral or pelvic corsets.
• Deep venous thromboses and pulmonary emboli, reduced muscle strength with prolonged recovery, postural hypotension and impaired cardiac function, atelectasis and pneumonia. Skin breakdown and pressure ulcers, constipation and fecal impaction, depression and intellectual regression are known complications of prolonged periods of inactivity.
• The overall 1-year mortality rate associated with pelvic insufficiency fractures is 14.3% and 50% of affected patients will not return to their prior level of function.
• Although initial clinical improvement may occur rapidly, compete resolution of symptoms may not occur for up to 9 to 12 months.
• Despite a favorable natural history, more aggressive treatments may benefit certain patients who are incapacitated by painful sacral insufficiency fractures.
Chronic symptoms and disability related to osteoporotic insufficiency fractures are believed to be due to fracture nonunion, micromotion, or resultant deformity related to the anemic attempts of the weakened bone to heal. The percutaneous injection of polymethylmethacrylate (PMMA) into fractured vertebral bodies (vertebroplasty) has been safely performed to successfully treat painful osteoporotic compression fractures. A natural extension in the application of vertebroplasty is the percutaneous injection of synthetic bone cement into the fractured sacrum (sacroplasty) to treat persistent symptoms and disability. Sacroplasty was first reported in 2001 as treatment of symptomatic sacral metastatic lesions, and subsequent reports have documented its safe and effective performance.
DIAGNOSIS
• Once suspicion of a sacral insufficiency fracture is suspected, then appropriate imaging is necessary. Some patient may already have had normal spinal or pelvic radiographs.
• The gold standard, which yields the highest sensitivity and specificity, is a magnetic resonance imaging (MRI). If a patient has a pacemaker or other condition that precludes obtaining an MRI, a computed tomography (CT) is necessary to compliment the bone scan. CT scans are more sensitive; however, nondisplaced fractures without reactive sclerosis may be missed.
• Fractures of the sacrum are best shown on coronal planes with a dedicated MRI of the sacrum.
• Traditionally, ordered MRI of the lumbar spine may miss a small percentage of fractures of the sacrum.
• Fat-suppressed T2-weighted or short T1 inversion recovery (STIR) sequences are the best imaging to diagnose these fractures (Figure 29-1). The most sensitive but not specific study is a bone scan, which may be positive for up to 1 year after the acute fracture. Honda sign, a typical H-shaped pattern on bone scan is pathognomonic and represents a bilateral sacral alar fracture with a horizontal fracture through the sacrum connecting them (Figure 29-2).
• A three-dimensional or bone scan with single photon emission CT (SPECT) may also aid in helping diagnose sacral insufficiency fractures.
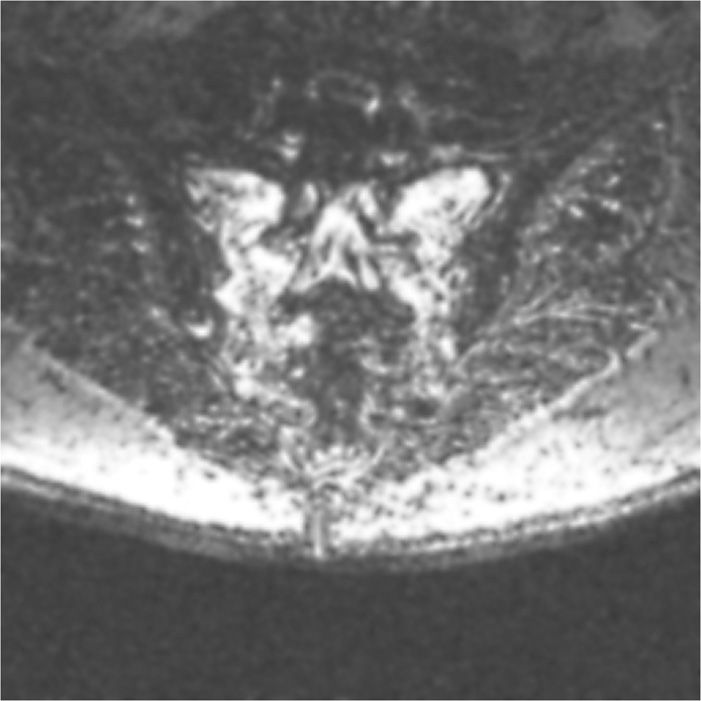
Figure 29-1. Axial view of a T2-fat suppression image demonstrating bilateral sacral insufficiency fractures.
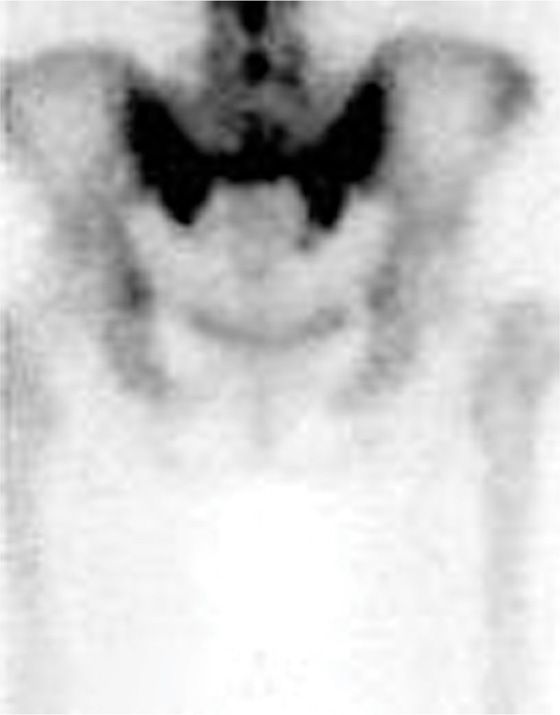
Figure 29-2. Honda sign on bone scan with SPECT imaging demonstrating bilateral sacral insufficiency fractures.
Fractures can occur in several places. The most common is a bilateral sacral ala fractures. Unilateral fractures can occur with or without a central horizontal component.
RELEVANT ANATOMY
Anatomically, the sacrum is comprised of 5 fused segments. Weight transfers can occur through the sacral segments which can allow a unique appearance of sacral fractures. In 1988, Denis et al classified the location of sacral fractures into 3 zones (Figure 29-3).
• Zone 1 involves the sacral ala but does not traverse the central sacral canal or foramen.
• Zone 2 involves sacral foramen but does not involve the central canal.
• Zone 3 involves the central canal. Patients with zone 3 fractures are associated with saddle anesthesia, loss of sphincter tone as a result of cauda equina injury.
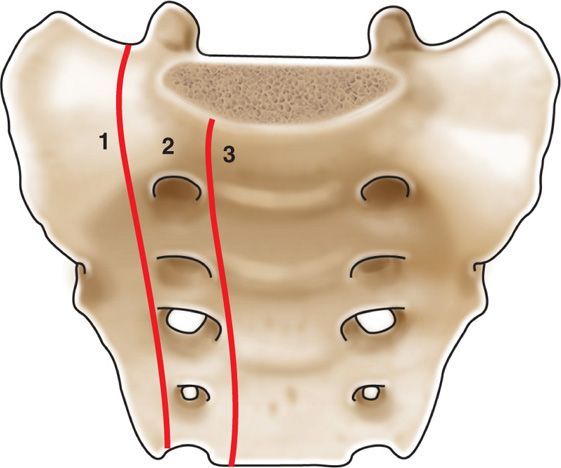
Figure 29-3. Denis classification of sacral fracture location.
The most common sacral insufficiency fractures are classified as zone 1. These fractures run parallel to the sacroiliac join along the entire sacral ala. When viewing sacral fractures via imaging techniques, place technical considerations when later deciding to perform sacral augmentation.
The key to successful sacroplasty depends on correctly identifying the following structures:
• Sacrum—including the posterior, anterior, and superior borders
• Four bilateral sacral foramen
• Sacroiliac joint
• Sacral canal
Other relevant that should be taken into consideration when performing Sacroplasty are:
• Rectum—this is separated from the ganglion by a layer of extraperitoneal fat and connective tissue
• Exiting nerve roots from the sacral foramen
• Sacral nerves traveling within the sacral canal
• Erector spinae muscles
BASIC CONCERNS AND CONTRAINDICATIONS
As with any intervention, one must weigh the ratio of potential benefits to the potential complications. In an average patient, and given the safety of the technique described here, sacroplasty should be considered early on in the treatment algorithm in the properly indicated patient. However, given the associated pathophysiology, one must carefully consider each patient individually.
Some basic concerns for injection are as follows:
• Immunocompromised patients are potentially at high risk for infection, this is of particular relevance in patients with malignancy.
• Metastatic cancer with local masses in the region.
• Thrombocytopenia or coagulopathy.
• Prone position may be difficult if patient has associated pelvic fractures.
• Possible rectal perforation if the needle is advanced too far anteriorly.
• Possible nerve damage if the needle is advanced into the sacral canal or foramen.
Contraindications for injection include:
• Infection, systemic or localized
• Coagulopathy
• Distorted or complicated anatomy
• Acute neurological deficits related to the fracture
• Patient refusal
PREOPERATIVE CONSIDERATIONS
• Informed consent and proper explanation of all potential complications.
• Anticoagulation—this is less of a concern than for an epidural injection but a concern nonetheless as there is inherent disruption of tissue from the introduction of a needle.
• Physical examination of the area for infection, skin ulceration, or necrosis.
• Patient must be able to lie prone for the intended length of the procedure.
• Intravenous access for IV fluid and medications for sedation or hypotension if the patient experiences vasovagal reaction or reaction to the PMMA cement.
Equipment
• Vertebroplasty trocars—13g or 11g
• PMMA cement and delivery system
• 5-cc syringe for medications
• 10-cc syringe for local anesthetic
• 1.5- to 3.5-in 25-gauge needle for local anesthetic
• 3.5-in spinal needles for placement in sacral foramen
Technique
• Sacroplasty is performed either by fluoroscopic guidance, computed tomography (CT) guidance, or a combination of both.
• When visualizing the sacrum under CT guidance, the choice of the entry site depends on the fracture lines.
• For H-shaped fractures, one may chose fixation of the horizontal component by accessing the needle puncture posterolaterally through the sacroiliac joint (Figure 29-4).
• Other alternative sites are punctures over the sacral ala with angulation of the needle between the spinal canal and the ipsilateral sacral foramen.
• The lateral fractures can be accessed by placing the trocar more medially and advancing the slightly laterally avoiding the sacral foramen and parallel to the fracture line.
• Sacroplasty may also be performed with fluoroscopic visualization safely if careful attention to landmarks and anatomic structures is followed.
• Needles may be placed in the lateral aspect of the sacral foramen to help aid in visualization of the foramen during the procedure. This describes a variation of the short axis technique using fluoroscopic visualization.
• The patient is placed in a prone position and after an oblique view aligning the entire sacroiliac joint, after local anesthetic infiltration, two 13-gauge vertebroplasty trocars are placed between the sacral foramen and sacroiliac joint on the side of the fractured ala at a 45-degree angle toward the sacroiliac joint.
• The trocars are then inserted approximately to midpoint of the sacrum, under lateral view, maintaining the 45-degree angle (Figure 29-5).
• After mixing the PMMA cement, under AP imaging, 2 to 5 cc of PMMA are injected through each trocar, monitoring the spread of the bone cement, to avoid medial extension toward the sacral nerve roots (Figure 29-6).
• An alternative method is to advance the trocar from the caudal aspect of the sacrum, through the body of the sacrum, lateral to the sacral foramen and medial to the SI joint.
• The trocar is advanced from the lower sacral segment toward the sacral ala. Fluoroscopic imaging in both lateral and AP views is necessary. In this manner, the trocar should be parallel to the vertical fractures (Figure 29-7). This allows for the PMMA cement to be deposited along the entire fracture line by injecting as the trocar is withdrawn.
• Once correct placement of the trocar is confirmed, PMMA cement is injected slowly and incrementally under constant fluoroscopic visualization (Figure 29-8).
• Constant vigilance to fluoroscopic imaging must occur to ensure cement extravasation does not occur.
• The patient should be maintained in the prone position for 10 to 20 minutes until the cement has set up and hardened. Postprocedure, patient should be monitored for neurological deficits, systemic or procedure related complications.
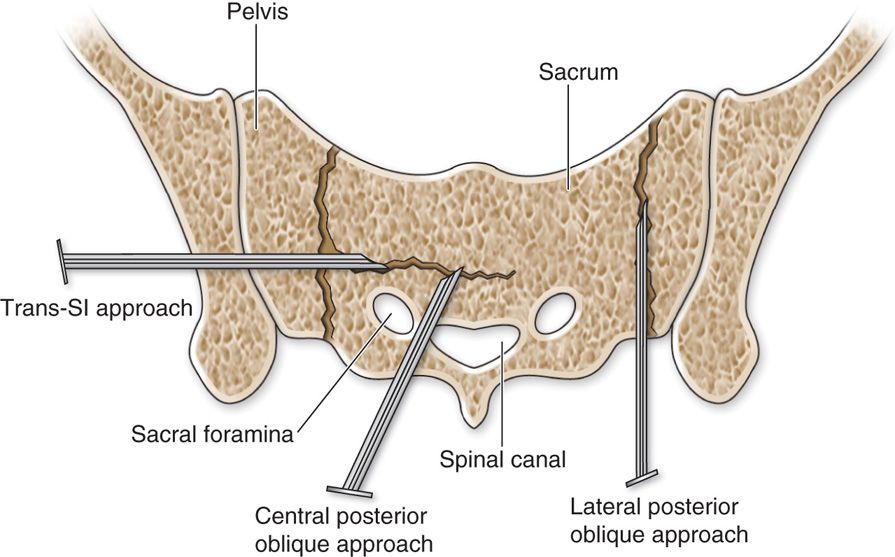
Figure 29-4. H-shaped fractures (Reprinted with the kind permission of Springer Science+Business Media from Mathis JM, Deramond H, Belkoff SM (eds). Percutaneous Vertebroplasty and Kyphoplasty, Second Edition. New York Springer Science+Business Media, 2006. Ch 16, Fig 16.7)
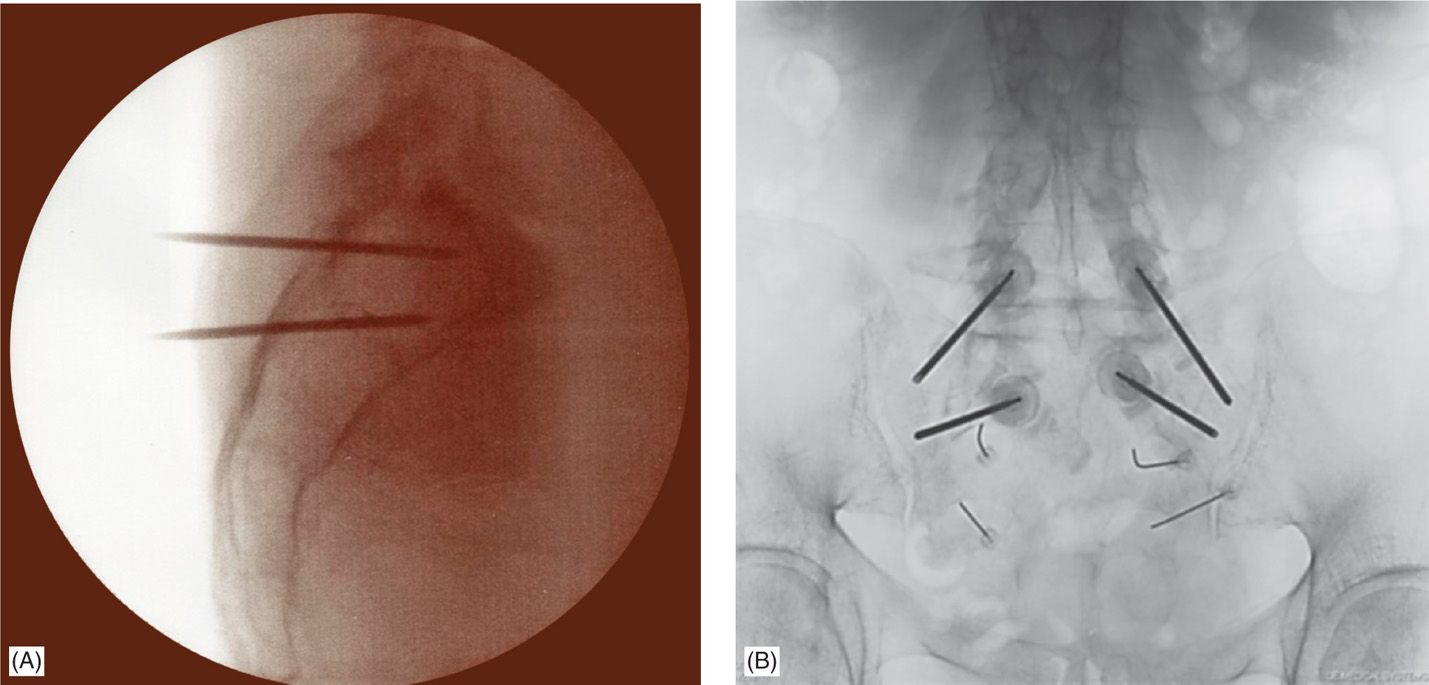
Figure 29-5. (A) Lateral fluoroscopic view of bilateral needle placement in the sacrum. (B) AP fluoroscopic view of bilateral needle placement.
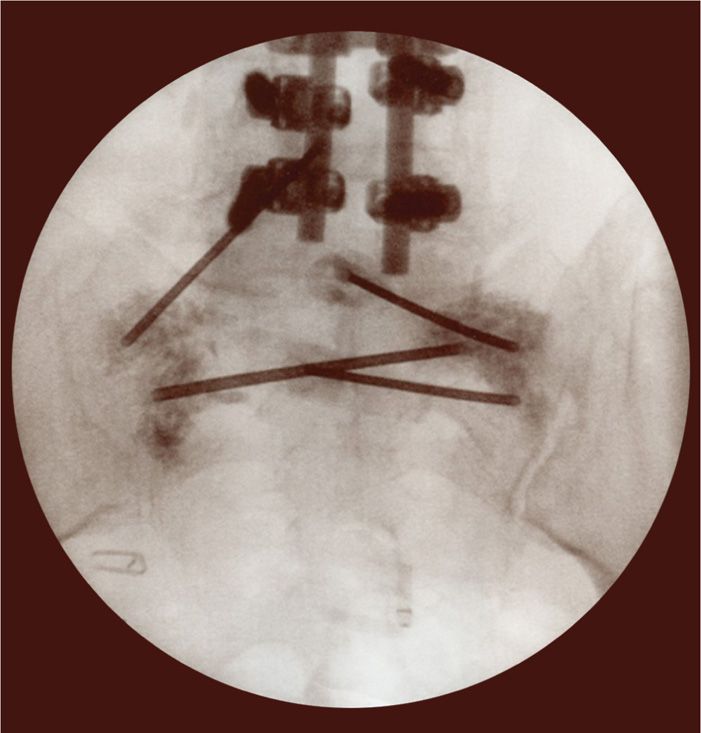
Figure 29-6. AP view of bilateral needle placement with cement placement to the bilateral sacral alae.
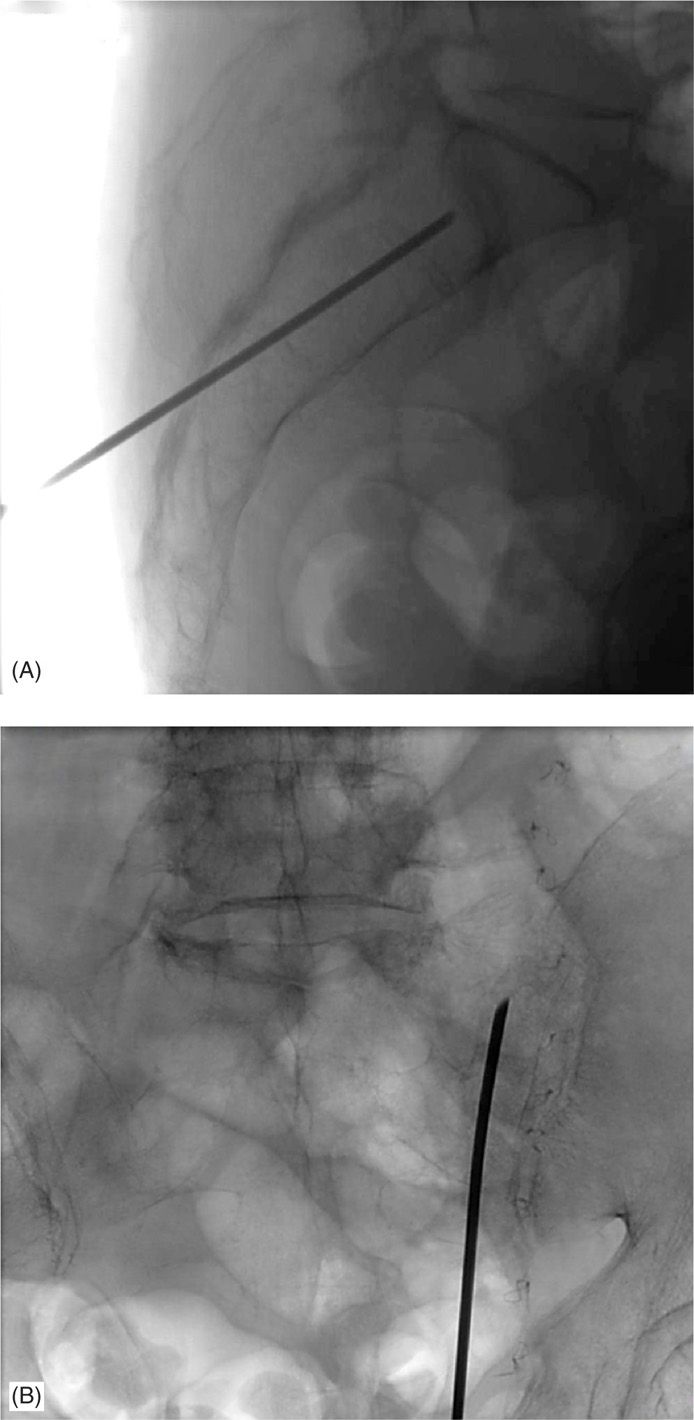
Figure 29-7. (A) Lateral view of the longitudinal approach under fluoroscopic guidance. (B) AP view of the longitudinal approach under fluoroscopic guidance.
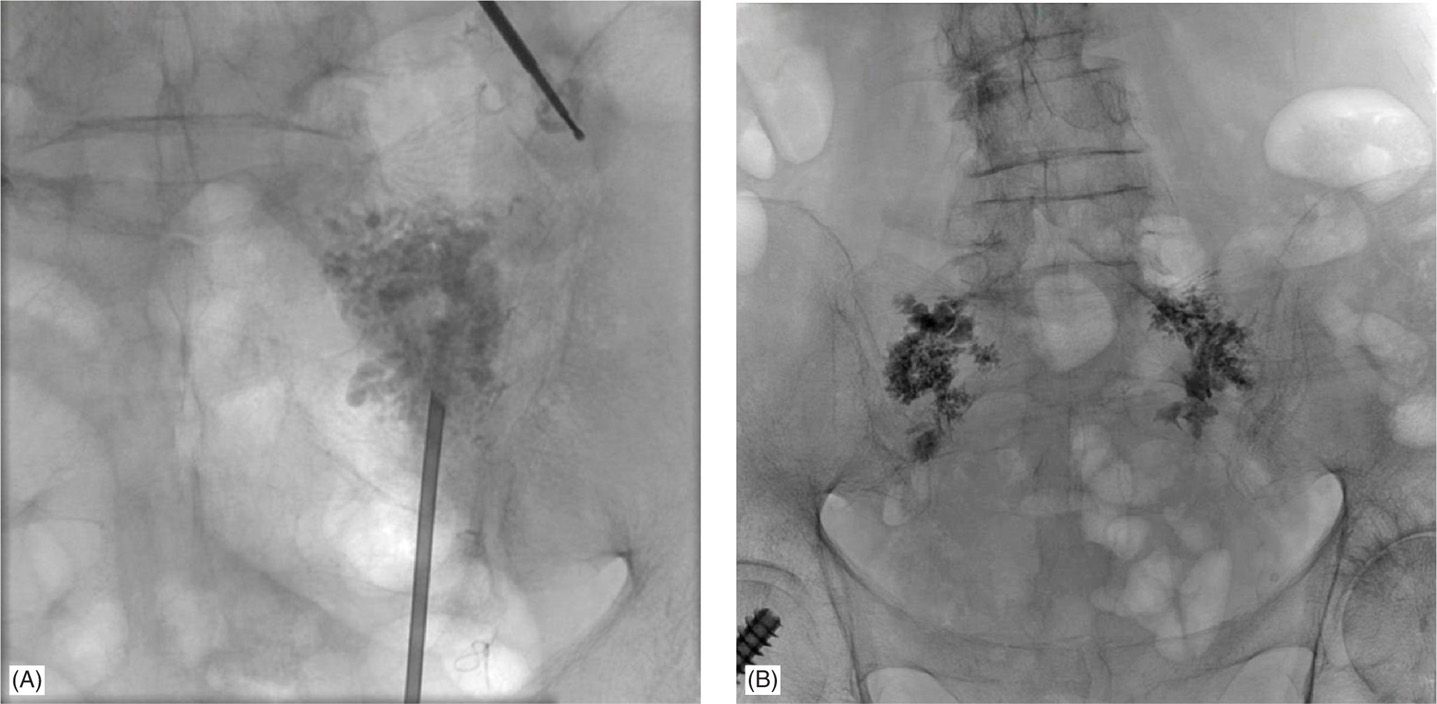
Figure 29-8. (A) AP view of cement placement with the longitudinal approach under fluoroscopic guidance. (B) AP view of bilateral cement placement with needles withdrawn (finished product).
POTENTIAL COMPLICATIONS
• Infection
• Bleeding
• Rectal perforation
• Cauda equina syndrome
• Nerve root or intraforaminal injection
• Intravascular injection
• Osteitis or periostitis
• Hematoma
• Inadvertent cement spread may potentially lead to motor, sexual, or bowel/bladder dysfunction
CLINICAL PEARLS
• When injecting, always start in AP to ensure cement is not spreading medially.
• Under lateral view, keep the needle in the middle third of the sacral segments to decease migration of cement.
• Always perform neurologic examination to make sure patient has adequate strength in the legs prior to discharge.
• MRI of the lumbar spine may not be sufficient. MRI of the sacrum is gold standard.
• If the patient cannot have an MRI, get CT and bone scan with SPECT imaging.
• When using the longitudinal approach, stay lateral to the neural foremen.
• Inject small incremental amounts of PMMA with frequent visualization in both AP and lateral views.
• If cement extravasation occurs, stop injecting. Allowing the cement to harden a little at that area may allow you to continue injecting in another area without further extravasation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







