Fig. 70.1
Percutaneous peripheral nerve stimulation lead
A variety of stimulators were used in the various studies during the developmental course of this technique, with the most recent version shown in Fig. 70.2. Stimulation parameters were tailored to the specific clinical application. Applications that require muscle contraction delivered a biphasic, charge balance waveform of 12 Hz at 20 mA, with intensity of muscle contraction modulated by adjusting the pulse duration between 20 and 200 μs. Applications that require sensory paresthesias delivered the same biphasic waveform but at 50 Hz and at 1–2 mA. The pulse duration was adjusted between 20 and 200 μs to modulate the intensity of the paresthesia.
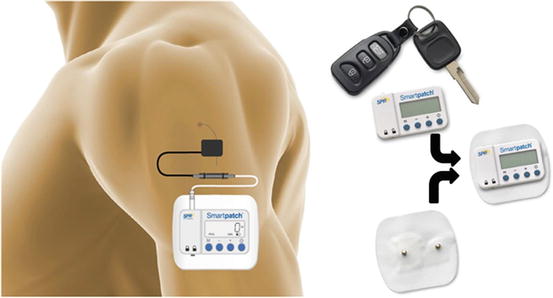

Fig. 70.2
Percutaneous peripheral nerve stimulator
Poststroke Shoulder Pain
PSSP is a debilitating complication of stroke, affecting a great majority of stroke survivors with moderate to severe hemiplegia. The glenohumeral joint is the most mobile joint in the human body; therefore, this joint is also the least stable, relying primarily on the rotator cuff for stability. With the onset of hemiparesis, the initial inciting cause of PSSP is glenohumeral instability. However, as pain transitions to the chronic phase, PSSP is likely mediated by central sensitization, a mechanism responsible for many chronic musculoskeletal pain conditions. PSSP is associated with depression, reduced motor and functional recovery, and poor quality of life.
PSSP was initially thought to be due to impaired glenohumeral biomechanics manifested by glenohumeral subluxation. Therefore, percutaneous PNS was initially developed to reduce glenohumeral subluxation and early trials only enrolled chronic stroke survivors with PSSP and glenohumeral subluxation. Leads were subcutaneously tunneled under local anesthesia and placed within the substance of the target muscle. Specifically, they were placed adjacent to the motor points of the supraspinatus, middle deltoid, and posterior deltoid to reduce the subluxation, and of the upper trapezius to stabilize the scapula. Participants were treated for 6 h daily for 6 weeks. Uncontrolled case series demonstrated significant reduction in pain and glenohumeral subluxation, which were sustained for up to 6 months after the end of treatment (EOT) [8, 9]. A follow-up multicenter randomized controlled trial (RCT) comparing PNS to a sling demonstrated significant pain reduction in the PNS group compared to the sling, with the treatment effect lasting for up to 12 months after EOT (Fig. 70.3) [10, 11].
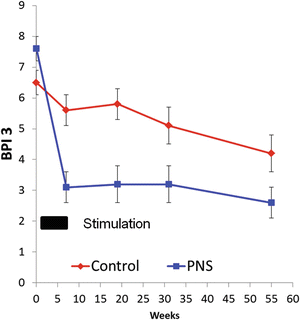

Fig. 70.3
Results of multicenter, single-blinded, RCT of percutaneous peripheral nerve stimulation vs. hemisling for the treatment of chronic poststroke shoulder pain
An important observation from the RCT, however, was the lack of significant reduction in glenohumeral subluxation, motor impairment, or hypertonia in the PNS group compared to controls. This suggested that improved biomechanics was not the mechanism responsible for the PNS-mediated pain reduction. Emerging data now suggest that while the pain mechanism during the early phase of PSSP is nociceptive, as pain transitions to chronic, central sensitization is the more likely mechanism sustaining the pain experience [12]. Thus, we now theorize that PNS reduces PSSP by the modulation of central sensitization. Accordingly, glenohumeral subluxation is no longer an inclusion criterion for this treatment protocol.
Other important changes were also implemented. Given that the improvement in biomechanics was unlikely to be the mechanism of action, the upper trapezius lead was removed from the protocol. Both the middle and posterior deltoids could be activated with a single lead. Thus, the 4-lead system evolved into a single-lead system. The reduction to a single lead obviated the need for tunneling and the introducer could now be inserted perpendicular to the skin surface, similar to the way needle EMG electrodes are placed. Review of the RCT data further revealed that most of the treatment effect was realized by the third week of stimulation. Thus, the duration of treatment was reduced by half. Overall, these changes have dramatically simplified the treatment protocol and have reduced the risk and discomfort to patients.
The revised treatment protocol was tested in two studies. The first study enrolled eight chronic stroke survivors with PSSP in an open-label case series [13]. Participants were treated with a single lead to the deltoid for 3 weeks and followed for 12 weeks. On average, the participants experienced 70%, 61%, and 63% pain reduction relative to baseline at EOT and at 4 and 12 weeks after EOT, respectively. In a follow-up single-blinded pilot trial [14], 25 chronic stroke survivors with PSSP were randomized to 3 weeks of percutaneous PNS or a course of physical therapy. As shown in Fig. 70.4, both groups experienced pain reduction by the EOT. However, the physical therapy group experienced pain recurrence, while the PNS group maintained their pain reduction. The revised protocol is presently undergoing a pivotal RCT in support of an application for FDA clearance for commercialization.
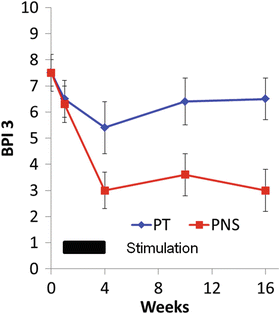

Fig. 70.4
Results of a single-site, single-blinded, RCT of percutaneous peripheral nerve stimulation vs. physical therapy for the treatment of chronic poststroke shoulder pain
Chronic Musculoskeletal Pain
The mechanism of percutaneous PNS-mediated pain reduction in PSSP is not known. However, the effect may be similar to the pain-reducing effect of exercise on chronic musculoskeletal pain [15]. We theorize that as muscles contract, the muscles themselves serve as a “translator” of information from the periphery to the central nervous system, the locus of maladaptive neuroplasticity of central sensitization. That is, as PNS induces comfortable muscle contractions, proprioceptors (Golgi tendon organ and muscle spindles) respond by sending physiologic information to the central nervous system, leading to modulation of central sensitization or dissensitization. This theory remains to be validated. There is now strong evidence that the convergent mechanism behind chronic musculoskeletal pain is central sensitization [16]. If percutaneous PNS reduces chronic PSSP by modulating central sensitization, and if all chronic musculoskeletal pain is mediated by central sensitization, then percutaneous PNS should reduce non-stroke-related chronic musculoskeletal pain.
To test this hypothesis, chronic SIS in the nonneurologically impaired population was selected as the test clinical condition. A 57-year-old male, with 20 months history of SIS, unresponsive to available conservative management, including ultrasound-guided subacromial corticosteroid injection, was implanted with a percutaneous lead to the deltoid and treated for 3 weeks [3]. His pain was reduced by 75% at the EOT and was pain free at 4 and 12 weeks after EOT. He reported similar improvements in pain interference with daily activities, and self-reports of function and quality of life. A follow-up open-label case series [17] among ten participants with chronic SIS showed significant reduction in pain and pain interference with daily activities (Fig. 70.5). This case series also reported preliminary evidence of PNS modulation of central sensitization.
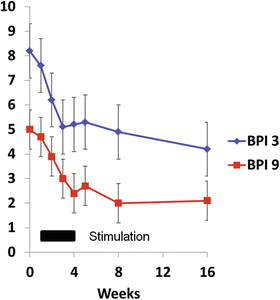

Fig. 70.5




Results of an open-label case series of percutaneous peripheral nerve stimulation for the treatment of chronic subacromial impingement syndrome
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





