PALLOR
SONAL N. SHAH, MD, MPH
Pallor, or the absence of skin coloration, is a relatively common problem in childhood. Throughout the world, the presence of pallor is often used as a screening tool to identify illness. The development of pallor can be acute and associated with a life-threatening illness, or it can be chronic and subtle, occasionally first noted by someone who sees the child infrequently. The onset of pallor can provoke anxiety for parents who are familiar with descriptions of the presentation of leukemia in childhood. In some instances, only reassurance may be needed, as in the case of a light complexioned or fair-skinned, nonanemic child. Even if there is a hematologic cause for the pallor, it is often a temporary condition readily amenable to therapy. However, pallor can portend a severe disease, and especially when acute in onset, can herald a true pediatric emergency for which rapid diagnosis and treatment are essential.
The degree of pallor depends on the concentration of hemoglobin in the blood and the distribution of blood in the blood vessels of the skin. Any condition that decreases the concentration of hemoglobin or alters the distribution of blood away from the body’s surface may present as pallor. Clinically, pallor caused by anemia can usually be appreciated when the hemoglobin concentration is below 8 to 9 g per dL, although the complexion of the child and the rapidity of onset may influence this value.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis for nonhematologic causes of pallor is outlined briefly in Table 57.1 and hematologic causes in Table 57.2. The concentration of hemoglobin in the blood can be lowered by three basic mechanisms: decreased erythrocyte or hemoglobin production, increased erythrocyte destruction, and blood loss. The most common causes of pallor and anemia seen in the emergency department (ED) are iron deficiency and blood loss (Table 57.3), but several less common diseases remain important considerations.
Decreased Production of Hemoglobin and Red Cells
Nutritional Anemias
Nutritional iron deficiency is the most common cause of decreased hemoglobin production in children. A peak in the prevalence of iron-deficiency anemia occurs between 12 and 24 months of age, when dietary iron content is often insufficient to meet the demands of a rapidly increasing red cell mass. Premature infants are more susceptible to developing iron-deficiency anemia because iron stores at birth are less than those found in term infants, whereas the growth (and therefore, expansion of the red cell mass) of the premature infant is often faster than that of term infants. The early exhaustion of iron stores in premature babies may result in pallor by 6 months of age, whereas in normal infants, signs of iron-deficiency anemia are uncommon before 10 to 12 months of age.
A thorough history and physical examination will provide important clues in the diagnosis of iron-deficiency anemia. History suggestive of a lack of iron in the diet may be readily apparent or may be recognized only after careful questioning, particularly regarding the daily consumption of cow’s milk. The infant with severe iron deficiency is often irritable and very pale. A compensatory increase in cardiac output is seen, which, when coupled with conditions that increase systemic demands on the heart (such as fever), may provoke the development of congestive heart failure (see Chapter 94 Cardiac Emergencies).
Serum hemoglobin concentration may be as low as 2 g per dL in severe iron-deficiency anemia. Red blood cells are markedly microcytic and hypochromic, and wide variation in red cell size and shape is usually present. Although the percentage of reticulocytes may be elevated moderately, the absolute reticulocyte count is low.
The diagnosis of iron deficiency as the cause for an anemia can often be made on the basis of the history alone, and treatment is usually instituted before confirmatory laboratory studies are available. Free erythrocyte protoporphyrin, a precursor to mature hemoglobin, is increased in iron-deficiency anemia and readily assayed. It can be a useful measure when evaluating the severely anemic child in the ED. Measurements of serum iron and ferritin levels have too long of a turnaround time to be of much value in the emergency management of anemia, but are valuable confirmatory tests. The concentration of hemoglobin in the reticulocyte (CHr) is one of the indices reported with reticulocyte counts, and serves as a sensitive marker of response to iron therapy at outpatient follow-up.
Other nutritional anemias, such as vitamin B12 or folic acid deficiency, are uncommon in children in the United States. When present, these anemias are likely associated with particular conditions such as a grossly altered diet, extended hyperalimentation, intestinal resection, or chronic diarrhea. Affected infants usually present with failure to thrive and developmental delay. Older patients more commonly exhibit weight loss, constipation, and weakness. The diagnosis of vitamin B12 or folic acid deficiency may be suggested by the finding of anemia with megaloblastic features. Megaloblastic anemia is characterized by normochromic, macrocytic red blood cells, hypersegmented neutrophils, and an elevated serum level of lactic dehydrogenase. The diagnosis is confirmed by the finding of low serum levels of folic acid or vitamin B12 and the response to folic acid or vitamin B12 replacement therapy.
TABLE 57.1
PALLOR WITHOUT ANEMIA

Hypoplastic and Aplastic Anemia
Pallor is usually the first sign of aplastic or hypoplastic anemia. These anemias may be congenital or acquired. Congenital aplastic anemias are most commonly part of larger syndromes, with the two major syndromes recognized being Diamond–Blackfan and Fanconi anemia.
Diamond–Blackfan syndrome is a congenital hypoplastic anemia commonly detected in the first few months of life. The anemia can be severe at the time of diagnosis. The red cells are normocytic or macrocytic. The reticulocyte count is characteristically low. Associated congenital anomalies include microcephaly, cleft palate, web neck, and thumb irregularities. The diagnosis is made by examination of a bone marrow aspirate evidencing markedly reduced or absent erythrocyte precursors with normal marrow cellularity.
TABLE 57.2
PALLOR WITH ANEMIA
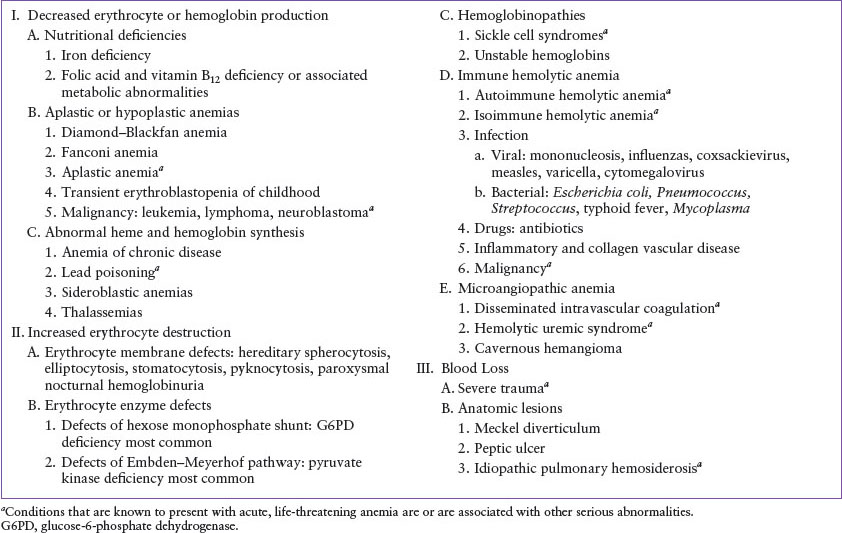
TABLE 57.3
RELATIVELY COMMON CAUSES OF PALLOR OR ANEMIA

Fanconi anemia is an autosomal recessive condition that results in progressive bone marrow failure generally after 3 or 4 years of age. Fanconi anemia is characterized by a normochromic or macrocytic anemia as well as reductions in both white cell and platelet counts, in contradistinction to Diamond–Blackfan syndrome. Other phenotypic abnormalities associated with Fanconi anemia include hyperpigmentation or hypopigmentation, microcephaly, strabismus, small stature, mental retardation, and anomalies of the thumbs and radii. The diagnosis may be made in the proper clinical context by the presence of increased chromosomal breakage in lymphocytes cultured in the presence of DNA cross-linking agents.
Acquired aplastic anemia can also present with severe pallor in children. The anemia is usually associated with granulocytopenia and thrombocytopenia. Acquired aplastic anemia is often idiopathic but has been associated with exposure to certain drugs and chemicals (e.g., chloramphenicol, felbamate, lindane, gold, benzene, and pesticides), radiation, and viral infections (especially hepatitis). The diagnosis is made by an examination of the bone marrow.
Transient erythroblastopenia of childhood (TEC) is a condition that is often associated with a recent viral illness and is characterized by moderate to severe anemia caused by diminished red cell production. The age at presentation can vary from infancy to 10 years, with a median of 18 to 26 months. The mean corpuscular volume (MCV) is usually normal at the time of diagnosis. The reticulocyte count is decreased, and a Coombs test is negative. The anemia of TEC may be associated with a normal or moderately decreased white cell count and a normal platelet count. Bone marrow examination shows an initial reduction or absence of erythrocytic precursors followed by erythroid hyperplasia during recovery. Transient erythroblastopenia that occurs in the first 6 months of life may be difficult to distinguish from Diamond–Blackfan anemia. Spontaneous recovery ultimately confirms the diagnosis of TEC.
Hypoplastic anemia can be the presenting symptom of childhood malignancies. The pallor can be severe, and although all three cell lines of the bone marrow are usually affected, anemia may be the only notable hematologic abnormality. The diagnosis can be suspected from the presence of other symptoms or findings, such as lymphadenopathy, bruising, limb pain, gum bleeding, or an abdominal mass.
Red cell aplasia may develop in patients with underlying hemolytic anemias such as hereditary spherocytosis or sickle cell disease (SCD), usually in association with parvovirus B19 infection. Decreased red cell production in the face of ongoing hemolysis causes an exacerbation of the anemia. The elevated reticulocyte count usually seen in hereditary hemolytic anemias falls to inappropriately low levels, often less than 1%. Although platelets and white cells are generally unaffected, they may be mildly decreased. Red cell transfusions are appropriate if the anemia is associated with cardiovascular compromise or if continuing reticulocytopenia indicates that the anemia is likely to become severe before the usual spontaneous recovery after 3 to 7 days. Patients with decreased erythrocyte production from a condition such as iron-deficiency anemia or human immunodeficiency virus (HIV) infection may also experience a transient aplastic crisis from parvovirus B19 infection. Hematologically normal children with underlying (though sometimes unrecognized) immunologic disorders may also develop parvovirus-induced anemia as a result of prolonged viremia.
Disorders of Heme and Globin Production
Pallor may be the presenting sign of nonnutritional disorders of hemoglobin synthesis, including the sideroblastic anemias and thalassemia syndromes. These disorders are characterized by a microcytic, hypochromic anemia with elevated reticulocyte counts.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







