Study objective
Recent-onset atrial fibrillation and flutter are the most common arrhythmias managed in the emergency department (ED). We evaluate the management and 30-day outcomes for recent-onset atrial fibrillation and flutter patients in Canadian EDs, where cardioversion is commonly practiced.
Methods
We conducted a prospective cohort study in 6 academic hospital EDs and enrolled patients who had atrial fibrillation and flutter onset within 48 hours. Patients were followed for 30 days by health records review and telephone. Adverse events included death, stroke, acute coronary syndrome, heart failure, subsequent admission, or ED electrocardioversion.
Results
We enrolled 1,091 patients with mean age 63.9 years, atrial fibrillation 84.7%, atrial flutter 15.3%, hospital admission 9.0%, and converted to sinus rhythm 80.1%. Although 10.5% of recent-onset atrial fibrillation and flutter patients had adverse events within 30 days, there were no related deaths and 1 stroke (0.1%). Adjusted odds ratios for factors associated with adverse event were hours from onset (1.03/hour; 95% confidence interval [CI] 1.01 to 1.05), history of stroke or transient ischemic attack (2.09; 95% CI 1.01 to 4.36), and pulmonary congestion on chest radiograph (7.37; 95% CI 2.40 to 22.64). Patients who left the ED in sinus rhythm were much less likely to experience an adverse event ( P <.001).
Conclusion
Although most recent-onset atrial fibrillation and flutter patients were treated aggressively in the ED, there were few 30-day serious outcomes. Physicians underprescribed oral anticoagulants. Potential risk factors for adverse events include longer duration from arrhythmia onset, previous stroke or transient ischemic attack, pulmonary congestion on chest radiograph, and not being in sinus rhythm at discharge. An ED strategy of sinus rhythm restoration and discharge in most patients is effective and safe.
Introduction
Background
Atrial fibrillation is characterized by disorganized atrial electrical depolarization leading to an irregular and rapid pulse rate. In the emergency department (ED), physicians often manage patients with either recent-onset or permanent (chronic) atrial fibrillation. In the case of permanent atrial fibrillation, cardioversion has previously failed or clinical judgment has led to a decision not to pursue cardioversion, with ED care focusing on rate control. When atrial fibrillation terminates spontaneously within 7 days of recognized onset, it is designated paroxysmal; when sustained beyond 7 days, atrial fibrillation is designated persistent. Atrial flutter is an arrhythmia with similar pathophysiology that is characterized by rapid, regular atrial depolarizations at a characteristic rate of approximately 300 beats/min and presents with various degrees of atrioventricular block. Atrial flutter is less common than atrial fibrillation but has similar management issues in the ED, and most patients with atrial flutter also have episodes of atrial fibrillation. Our focus is on symptomatic patients with recent-onset atrial fibrillation and flutter, ie, those with episodes of atrial fibrillation or atrial flutter (first detected, recurrent paroxysmal, or recurrent persistent) in which the onset is less than 48 hours and cardioversion is an option. Recent-onset atrial fibrillation and flutter are the most common acute arrhythmia cases requiring care in the ED.
What is already known on this topic
Recent-onset atrial fibrillation and flutter are commonly treated in the emergency department (ED).
What question this study addressed
What happens to patients with these 2 rhythms in a setting in which ED cardioversion attempts (electrical and pharmacologic) are common?
What this study adds to our knowledge
In a 1,091-subject cohort from 6 Canadian EDs, 80.1% converted to sinus rhythm. Adverse effects in the next 30 days included only 1 stroke and no deaths.
How this is relevant to clinical practice
ED cardioversion of these patients often succeeds without harm, buttressing the argument for embracing this practice.
Importance
Variation in practice within EDs has been well described and reflects a lack of high-quality evidence to guide the acute management of recent-onset atrial fibrillation and flutter. Standard textbooks and guidelines fail to offer clear evidence-based direction for physicians treating recent-onset atrial fibrillation and flutter. Particularly controversial is the issue of using rhythm control or rate control. The large Atrial Fibrillation Follow-up Investigation of Rhythm Management and AF-CHF trials compared rate and rhythm control for patients with mostly recurrent, persistent atrial fibrillation but did not explore the optimal management for ED recent-onset atrial fibrillation and flutter patients presenting within 48 hours of onset. In the United States, patients are often admitted to the hospital under the cardiology service or discharged home after rate control only. In Canada, emergency physicians are much more likely to follow an aggressive antiarrhythmia treatment approach using pharmacologic cardioversion or electrocardioversion. They perceive that this strategy has significant benefits for patients: immediate return to normal activities without the need for hospital admission or need for treatment with rate control and oral anticoagulant drugs. Two sites have described several cohorts of patients successfully treated with rhythm control, with good results. Other ED studies of rhythm control for recent-onset atrial fibrillation and flutter have been small or did not include both pharmacologic and electrocardioversion as an option.
Goals of This Investigation
We are not aware of previous studies that prospectively followed recent-onset atrial fibrillation and flutter patients after ED disposition. We sought to fill this knowledge gap about the outcomes and adverse events that might occur in such patients after a sentinel ED visit, regardless of initial management or disposition. In particular, our goal was to describe ED management and then follow patients prospectively for 30 days to determine clinical outcomes, use of health care resources, use of oral anticoagulants, and adverse events. Finally, we wished to evaluate potential risk factors for these adverse events to better understand how to prevent them.
Materials and Methods
Study Design and Setting
We conducted a prospective cohort study in 6 Canadian academic hospital EDs.
Selection of Participants
We attempted to enroll consecutive patients presenting with an episode of recent-onset atrial fibrillation and flutter, in which symptoms required urgent management and in which pharmacologic or electrocardioversion was an option. Specifically, we included patients with a clear history of onset within 48 hours, or a clear history of onset within 7 days and who had received adequate anticoagulation, or a clear history of onset within 7 days and no left atrial thrombus on transesophageal echocardiography. We did not exclude patients who required admission or who converted spontaneously to sinus rhythm before treatment.
We excluded patients who had been previously enrolled, with permanent or persistent atrial fibrillation, or whose primary presentation was for another condition such as (1) acute coronary syndrome presenting with chest pain and acute ischemic changes on ECG; (2) congestive heart failure with severe shortness of breath requiring immediate intravenous diuretic, nitrates, or bilevel positive airway pressure; (3) pneumonia with temperature greater than 38.5°C (101.3°F), respiratory symptoms, and receiving antibiotics in the ED; (4) pulmonary embolism presenting with chest pain or shortness of breath; and (5) sepsis with infection and 2 or more systemic inflammatory response syndrome criteria.
Patients were identified prospectively in the ED and then followed by telephone interviews.
Patients gave consent to participate in the study, as approved by the respective hospital research ethics boards.
Methods of Measurement and Data Collection and Processing
The sources of data were the ED health record (including nursing and physician notes), hospital electronic records (clinical, laboratory, and imaging), ED enrollment form, clinic records, self-administered patient questionnaire, follow-up telephone interviews, and provincial coroners’ records. We collected extensive demographic and clinical patient data, details of ED treatment, outcomes, and disposition. The chest radiography interpretations were those of certified radiologists who had no knowledge of the study protocol. We then followed patients for 30 days to determine subsequent ED and physician visits, investigations and prescriptions, and need for cardioversion or admission. Site study staff were individually trained and monitored by a central study coordinator who reviewed source documents for the accuracy of the data submitted.
Outcome Measures
We were particularly interested in the occurrence of serious adverse events and their relationship with atrial fibrillation or atrial flutter. We created a composite outcome, serious event, that included the following within 30 days: death, stroke, acute coronary syndrome, heart failure, subsequent hospital admission related to atrial fibrillation or atrial flutter, and subsequent need for ED electrocardioversion.
Primary Data Analysis
Management, ED clinical outcomes, 30-day outcomes, and health care resource use were presented descriptively as appropriate for continuous, ordinal, and categorical outcomes. We classified the following as adverse events: death, stroke, acute coronary syndrome, acute heart failure, subsequent hospital admission related to atrial fibrillation or atrial flutter, and subsequent need for ED electrocardioversion. We evaluated the univariate association of 20 clinical and demographic factors with adverse events, using t tests, Mann-Whitney U tests, and χ 2 tests for continuous, ordinal, and categorical variables, respectively. We then conducted multivariate logistic regression analyses to identify independent predictors associated with adverse events. Model building proceeded with backward elimination selection P <0.1. The following independent variables were tested in the multivariate models: age, CHADS2 score, previous stroke, atrial fibrillation versus atrial flutter, hours since onset of atrial fibrillation and flutter, ischemia on ECG, congestion on chest radiograph, pulse rate at disposition from ED, and rhythm at disposition with mode of conversion (spontaneous, pharmacologic, or electrical). We estimated that approximately 1,000 patients would yield at least 100 adverse events, allowing us to evaluate at least 10 predictor variables in the multivariate modeling.
Materials and Methods
Study Design and Setting
We conducted a prospective cohort study in 6 Canadian academic hospital EDs.
Selection of Participants
We attempted to enroll consecutive patients presenting with an episode of recent-onset atrial fibrillation and flutter, in which symptoms required urgent management and in which pharmacologic or electrocardioversion was an option. Specifically, we included patients with a clear history of onset within 48 hours, or a clear history of onset within 7 days and who had received adequate anticoagulation, or a clear history of onset within 7 days and no left atrial thrombus on transesophageal echocardiography. We did not exclude patients who required admission or who converted spontaneously to sinus rhythm before treatment.
We excluded patients who had been previously enrolled, with permanent or persistent atrial fibrillation, or whose primary presentation was for another condition such as (1) acute coronary syndrome presenting with chest pain and acute ischemic changes on ECG; (2) congestive heart failure with severe shortness of breath requiring immediate intravenous diuretic, nitrates, or bilevel positive airway pressure; (3) pneumonia with temperature greater than 38.5°C (101.3°F), respiratory symptoms, and receiving antibiotics in the ED; (4) pulmonary embolism presenting with chest pain or shortness of breath; and (5) sepsis with infection and 2 or more systemic inflammatory response syndrome criteria.
Patients were identified prospectively in the ED and then followed by telephone interviews.
Patients gave consent to participate in the study, as approved by the respective hospital research ethics boards.
Methods of Measurement and Data Collection and Processing
The sources of data were the ED health record (including nursing and physician notes), hospital electronic records (clinical, laboratory, and imaging), ED enrollment form, clinic records, self-administered patient questionnaire, follow-up telephone interviews, and provincial coroners’ records. We collected extensive demographic and clinical patient data, details of ED treatment, outcomes, and disposition. The chest radiography interpretations were those of certified radiologists who had no knowledge of the study protocol. We then followed patients for 30 days to determine subsequent ED and physician visits, investigations and prescriptions, and need for cardioversion or admission. Site study staff were individually trained and monitored by a central study coordinator who reviewed source documents for the accuracy of the data submitted.
Outcome Measures
We were particularly interested in the occurrence of serious adverse events and their relationship with atrial fibrillation or atrial flutter. We created a composite outcome, serious event, that included the following within 30 days: death, stroke, acute coronary syndrome, heart failure, subsequent hospital admission related to atrial fibrillation or atrial flutter, and subsequent need for ED electrocardioversion.
Primary Data Analysis
Management, ED clinical outcomes, 30-day outcomes, and health care resource use were presented descriptively as appropriate for continuous, ordinal, and categorical outcomes. We classified the following as adverse events: death, stroke, acute coronary syndrome, acute heart failure, subsequent hospital admission related to atrial fibrillation or atrial flutter, and subsequent need for ED electrocardioversion. We evaluated the univariate association of 20 clinical and demographic factors with adverse events, using t tests, Mann-Whitney U tests, and χ 2 tests for continuous, ordinal, and categorical variables, respectively. We then conducted multivariate logistic regression analyses to identify independent predictors associated with adverse events. Model building proceeded with backward elimination selection P <0.1. The following independent variables were tested in the multivariate models: age, CHADS2 score, previous stroke, atrial fibrillation versus atrial flutter, hours since onset of atrial fibrillation and flutter, ischemia on ECG, congestion on chest radiograph, pulse rate at disposition from ED, and rhythm at disposition with mode of conversion (spontaneous, pharmacologic, or electrical). We estimated that approximately 1,000 patients would yield at least 100 adverse events, allowing us to evaluate at least 10 predictor variables in the multivariate modeling.
Results
Characteristics of Study Subjects
We enrolled 1,091 of 1,120 eligible patients between June 2010 and May 2012 at 6 hospital sites ( Figure ). Twenty-nine patients were missed, usually after hours, but we could detect no bias in patient selection. By review of electronic health and coroners’ records, we were able to ascertain the outcomes of all patients.
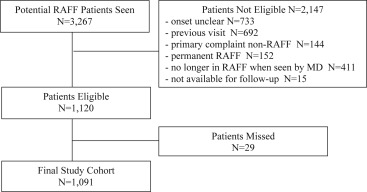
These recent-onset atrial fibrillation and flutter patients were younger than typical permanent atrial fibrillation patients, with a mean age of 63.9 years ( Table 1 ), although 17.2% were aged 80 years or older. On arrival to the ED, 84.7% of patients were in atrial fibrillation and 15.3% in atrial flutter, the mean duration of symptoms was 7.7 hours, and 65.0% had previous episodes of recent-onset atrial fibrillation and flutter. Of 630 patients (57.8%) with a CHADS2 score of 1 or more, only 202 (32.1%) were receiving warfarin. Although 73.6% of patients had troponin levels and 29.9% had thyroid-stimulating hormone levels measured, only 3 underwent transesophageal echocardiography while in the ED.
| Characteristic | Patients |
|---|---|
| Age, mean (SD), y | 63.9 (15.2) |
| Range | 19–103 |
| Men (%) | 649 (59.5) |
| Hospital (%) | |
| Kingston General Hospital, Kingston, ON | 152 (13.9) |
| Ottawa Hospital–Civic Campus, Ottawa, ON | 296 (27.1) |
| Ottawa Hospital–General Campus, Ottawa, ON | 182 (16.7) |
| University of Alberta Hospital, Edmonton, AB | 132 (12.1) |
| Foothills Medical Centre, Calgary, AB | 261 (23.9) |
| Mount Sinai Hospital, Toronto, ON | 68 (6.2) |
| Initial rhythm (%) | |
| Atrial fibrillation | 924 (84.7) |
| Atrial flutter | 167 (15.3) |
| Duration of arrhythmia, mean (SD) | |
| Hours (less than 48 h), N=1,052 | 7.7 (9.6) |
| Range | 1.0–48 |
| Days (between 3 and 7 days), N=39 ∗ | 4.0 (1.8) |
| Range | 2–7 |
| Main presenting symptom (%) | |
| Palpitations | 852 (78.1) |
| Chest pain | 127 (11.6) |
| Shortness of breath | 44 (4.0) |
| Dizziness | 37 (3.4) |
| Weakness | 11 (1.0) |
| Syncope | 12 (1.1) |
| Other | 8 (0.7) |
| Initial vital signs, mean (SD) | |
| Pulse rate | 120 (29) |
| Systolic blood pressure | 131 (23) |
| Oxygen saturation % | 98 (2) |
| Canadian Triage and Acuity Scale Level, median (IQR) † | 2 (0) |
| Previous atrial fibrillation (%) | 709 (65.0) |
| Electrocardioversion | 351 (32.2) |
| Pharmacologic cardioversion | 201 (18.4) |
| Ablation | 89 (8.2) |
| CHADS2 criteria (%) | |
| >75 y | 301 (27.6) |
| Stroke/transient ischemic attack | 76 (7.0) |
| Hypertension | 465 (42.6) |
| Diabetes mellitus | 98 (9.0) |
| Congestive heart failure | 50 (4.6) |
| CHADS2 score, median (IQR) ‡ | 1 (2) |
| Score >1 | 630 (57.8) |
| Receiving warfarin, N=630 | 202 (32.1) |
| Other medical history (%) | |
| Coronary artery disease | 194 (17.8) |
| Valvular heart disease | 92 (8.4) |
| Pacemaker/ICD | 39 (3.6) |
| COPD/asthma | 104 (9.5) |
| Current medications (%) | |
| β-Blockers | 432 (39.6) |
| Acetylsalicylic acid | 390 (35.8) |
| Warfarin | 278 (25.5) |
| Calcium-channel blocker | 199 (18.2) |
| Sotalol | 50 (4.6) |
| Clopidogrel | 46 (4.2) |
| Amiodarone | 45 (4.1) |
| Propafenone | 40 (3.7) |
| Digoxin | 33 (3.0) |
| Procainamide | 1 (0.1) |
| Investigations | |
| ECG shows ischemic changes (%) | 26 (2.4) |
| Initial ECG-calculated pulse rate, median, range | 125 (45–213) |
| Chest radiograph shows CHF (%) | 24 (2.2) |
| International normalized ratio (%) | 853 (78.2) |
| Level, mean, N=853 | 1.4 |
| Troponin level (%) | 803 (73.6) |
| Above 99th percentile (%), N=803 | 680 (84.7) |
| TSH (%) | 327 (30.0) |
| Below reference value (%), N=326 | 9 (2.8) |
| C-reactive protein (%) | 4 (0.4) |
| Level, mean, mg/L | 1.6 |
| Echocardiography (%) | |
| Transesophageal | 3 (0.3) |
| Transthoracic | 4 (0.4) |
| Left atrial clot | 0 |
| Significant valvulopathy | 1 (0.1) |
| Other conditions identified in ED (%) | |
| Congestive heart failure | 19 (1.7) |
| Acute coronary syndrome | 12 (1.1) |
∗ Patients fully anticoagulated or negative transesophageal echocardiogram result.
† Canadian Triage and Acuity Scale ranges from 1 (critical) to 5 (not urgent).
Main Results
Patients were most likely to be primarily treated with electrocardioversion or pharmacologic cardioversion (72.8%), with intravenous procainamide being by far the most common drug used ( Table 2 ). Electrocardioversion (97.9%) and sedation (98.3%) were almost always provided by the emergency physician. Heparin was rarely administered in the ED (4.6%). Adverse events with cardioversion were uncommon and usually transient ( Table 3 ).
| Treatment | Patients (N=1,091) |
|---|---|
| First attempted treatment (%) | |
| Observe only | 102 (9.4) |
| Rate control only | 194 (17.8) |
| Rhythm drug first | 368 (33.7) |
| Electrocardioversion first | 427 (39.1) |
| Second attempted treatment (%), N=167 | |
| Rhythm drug | 23 (13.8) |
| Electrocardioversion | 144 (86.2) |
| IV rate control drugs in ED (%) | 438 (40.2) |
| Metoprolol | 285 (65.1) |
| Total dose administered, mean, mg | 9 |
| Pulse rate 1 h post, mean | 102 |
| Diltiazem | 141 (32.2) |
| Total dose administered, mean, mg | 21 |
| Pulse rate 1 h post, mean | 97 |
| Other medications (%) | 12 (2.7) |
| Digoxin | 4 (0.9) |
| Bisoprolol | 2 (0.5) |
| Sotalol | 2 (0.5) |
| Atenolol | 1 (0.2) |
| Carvedilol | 1 (0.2) |
| Labetalol | 1 (0.2) |
| Esmolol | 1 (0.2) |
| IV adenosine administered (%) | 34 (3.1) |
| Rhythm control drugs in ED (%) | 391 (35.8) |
| Procainamide IV | 332 (84.9) |
| Amiodarone IV | 37 (9.5) |
| Propafenone PO | 11 (2.8) |
| Vernakalant IV | 4 (1.0) |
| Other medications | 9 (2.3) |
| Flecainide PO | 3 (0.8) |
| Dronedarone PO | 2 (0.5) |
| Ibutilide IV | 1 (0.3) |
| Successful conversion | 204 (52.2) |
| Electrocardioversion attempted (%) | 571 (52.3) |
| Successful conversion | 514 (90.0) |
| Max energy, mean, Joules | 148.0 |
| No. of shocks administered, mean | 1.4 |
| Pad position, N=56 | |
| Anterolateral | 4 (7.1) |
| Anteroposterior | 52 (92.9) |
| Physician performing cardioversion, N=571 | |
| Emergency physician | 559 (97.9) |
| Cardiology | 12 (2.1) |
| Sedation given by, N=571 | |
| Emergency physician | 561 (98.3) |
| Anesthesia | 9 (1.6) |
| Sedation used, N=571 | |
| Propofol | 549 (96.1) |
| Fentanyl | 316 (55.3) |
| Midazolam | 23 (4.0) |
| Other | 34 (6.0) |
| Second electrocardioversion required after recurrence | 11 (1.9) |
| Consultations in ED (%) | |
| Cardiology | 216 (19.8) |
| Internal medicine | 21 (1.9) |
| Anesthesia | 5 (0.5) |
| Antithrombotic therapy given in ED (%) | |
| Heparin | 50 (4.6) |
| IV unfractionated | 15 (1.4) |
| SC low molecular weight | 35 (3.3) |
| Warfarin ∗ | 40 (3.7) |
| Acetylsalicylic acid | 95 (8.7) |
| Clopidogrel | 8 (0.7) |
∗ No novel oral anticoagulants were used in the ED during the study period.
| Adverse Events (%) | Patients |
|---|---|
| If rhythm control drugs administered, N=391 | 42 (10.7) ∗ |
| Hypotension | 24 (6.1) |
| Drug infusion stopped | 15 (3.8) |
| Bradycardia | 12 (3.1) |
| Other | 6 (1.5) |
| Ventricular tachycardia | 4 (1.0) |
| Atrial tachyarrhythmia | 3 (0.8) |
| Heart block | 0 |
| Torsades de pointes | 0 |
| Syncope | 0 |
| Supraventricular tachycardia | 0 |
| If electrocardioversion attempted, N=571 | 19 (3.3) |
| Transient hypoxia | 19 (3.3) |
| Aspiration | 0 |
| Stroke | 0 |
| Death | 0 |
Only 9.0% of patients were admitted and only 19.9% were not in sinus rhythm at discharge ( Table 4 ). Although physician follow-up was routinely recommended, rarely was an outpatient echocardiogram ordered (8.2%) or oral anticoagulants prescribed (4.8%).
| Disposition Details (%) | Patients, N=1,091 |
|---|---|
| Disposition | |
| Discharged home | 993 (91.0) |
| Scheduled return to ED next day, N=993 | 21 (2.1) |
| Admitted | 98 (9.0) |
| Converted to sinus rhythm before ED discharge, N=1,089 ∗ | (80.1) |
| Electrical | 513 (47.1) |
| Drug | 204 (18.7) |
| Spontaneous | 155 (14.2) |
| Not converted | 217 (19.9) |
| Pulse rate before discharge, mean, beats/min | 76 |
| ED length of stay, hours, median (IQR), h | 5.0 (4) |
| Outpatient follow-up recommended N=993 † | |
| Cardiology | 630 (63.4) |
| Family physician | 413 (41.6) |
| Internal medicine | 29 (2.9) |
| Echocardiogram | 81 (8.2) |
| New prescriptions at discharge, N=993 | |
| Acetylsalicylic acid | 115 (11.6) |
| Metoprolol | 62 (6.2) |
| Warfarin ‡ | 48 (4.8) |
| Other cardiac medication | 41 (4.1) |
| Diltiazem | 21 (2.1) |
| Low molecular weight heparin | 12 (1.2) |
| Clopidogrel | 1 (0.1) |
∗ The mode of achieving discharge rhythm was unknown for 2 patients.
† Patients may have had more than 1.
‡ No novel oral anticoagulants were used in ED during the study period.
We successfully followed patients for 30 days and noted that 27.9% returned to the ED and 15.4% returned for an issue directly related to atrial fibrillation or atrial flutter ( Table 5 ). By 30 days, 50.7% of patients had consulted a physician and only small numbers of patients had received prescriptions for warfarin (4.5%) or novel oral anticoagulants (4.1%). We estimate that by 30 days, only 49.3% of patients with CHADS2 score of 1 or more were receiving oral anticoagulants.
| Outcome | Patients (N=1,091) |
|---|---|
| Return ED visit (%) | 304 (27.9) |
| Related to AF/AFL | 168 (15.4) |
| No. of visits, mean, N=168 | 1.5 |
| Days post ED, mean (SD), N=168 | 10.7 (8.3) |
| Outpatient visits (%) | |
| Cardiology follow-up | 278 (25.5) |
| No. of visits, mean | 1.2 |
| Days post ED, mean (SD) | 14.9 (9.0) |
| Internal medicine | 55 (5.0) |
| No. of visits, mean | 1.4 |
| Days post ED, mean (SD) | 9.8 (7.8) |
| Family physician | 269 (24.7) |
| No. of visits, mean, N=264 | 1.5 |
| Days post ED, mean (SD), N=269 | 9.2 (8.0) |
| Hospital admission (%) | 42 (3.9) |
| Related to atrial-fibrillation/flutter | 35 (3.2) |
| Days post ED, mean (SD), N=42 | 11.6 (8.0) |
| Length of stay, mean days (SD), N=39 | 6.9 (9.9) |
| Electrocardioversion (%) | 71 (6.5) |
| Days post ED, mean (SD) | 12.5 (8.6) |
| In ED | 61 (5.6) |
| In clinic | 10 (0.9) |
| Successfully cardioverted (%), N=71 | 62 (87.3) |
| Electrocardiography (%) | 401 (36.8) |
| Days post ED, mean (SD) | 15.1 (9.0) |
| Rhythm, N=374 | |
| Normal sinus | 296 (79.1) |
| Atrial fibrillation | 66 (17.7) |
| Atrial flutter | 12 (3.2) |
| Pulse rate, mean, N=338 | 73.2 |
| Other arrhythmia, N=1,091 | |
| AV block | 1 (0.1) |
| Ventricular tachycardia | 2 (0.2) |
| Supraventricular tachycardia | 3 (0.3) |
| Echocardiography (%) | 324 (29.7) |
| Transthoracic | 280 (86.4) |
| Transesophageal echocardiography | 20 (6.2) |
| Results of echocardiography, N=265 | |
| Left atrial clot | 0 |
| Significant valvulopathy | 48 (18.1) |
| Left atrial enlargement | 76 (28.7) |
| Left ventricular hypertrophy | 14 (5.3) |
| New medications prescribed (%) ∗ | 265 (24.3) |
| β-Blocker | 89 (8.2) |
| Calcium-channel blocker | 31 (2.8) |
| Antiarrhythmic | 86 (7.9) |
| Digoxin | 18 (1.6) |
| Warfarin | 49 (4.5) |
| Novel oral anticoagulant | 45 (4.1) |
| Acetylsalicylic acid | 30 (2.8) |
| Clopidogrel | 6 (0.6) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







