INTRODUCTION
Opioids refers broadly to all compounds related to opium that possess analgesic and sedative properties. Opiate describes the opioid alkaloids found naturally in the opium poppy plant, Papaver somniferum. The term narcotic refers to a broader group of agents, predominantly used by law enforcement to designate a variety of controlled substances with abuse or addictive potential; use of this term in medical practice is discouraged.
Opioid abuse is a significant public health issue in the United States with a dramatic increase in prescription opioid use and abuse in the past 10 years.1,2 Opioids most frequently involved in reported toxic drug exposures were, in order of number of cases recorded, tramadol, oxycodone, methadone, morphine, buprenorphine, and hydrocodone.3 Deaths were primarily associated with exposure to methadone, oxycodone, and morphine. The majority of prescription opioid overdose deaths were associated with diversion, doctor shopping, and nonmedical use.
PHARMACOLOGY
Opioids modulate nociception in the terminals of afferent nerves in the CNS, peripheral nervous system, and GI tract. Opioids are agonists at the three primary opioid receptors: μ (mu), κ (kappa), and δ (delta). Opioid receptors are similar to other G protein–coupled receptors; they are transmembrane proteins that undergo conformational change when activated by external molecules, and this change then alters some aspect of intracellular function. Opioid receptors vary widely in morphology and distribution. Also, the specificity and affinity of an opioid for a particular receptor are variable. For example, tramadol possesses 1/6000 the affinity of morphine at the μ-receptor site.
Stimulation of the μ-receptors results in analgesia, sedation, miosis, respiratory depression, cough suppression, euphoria, and decreased GI motility. Stimulation of κ-receptors results in weaker analgesia, sedation, miosis, decreased intestinal motility, dysphoria, and hallucinations. Stimulation of the δ-receptors results in some analgesia and antidepressant effect. All currently available opioid agonists possess μ-receptor activity and result in some degree of respiratory depression.
There is interplay between opioid receptors and other transmembrane receptors found in the nervous system. One example is that opioid binding to μ-receptors in the nucleus accumbens results in the localized release of dopamine (the “dopamine pleasure pathway”). A second example is that the analgesic effect of morphine is enhanced in the presence of N-methyl-d-aspartate receptor blockers such as amantadine. A third example is the induction of mast cell histamine release by morphine and meperidine.
Opioids can be categorized as naturally occurring compounds (termed opiates), chemical modifications of natural compounds (semisynthetic), and completely artificial compounds (synthetic) (Table 186-1). Some opioids are agonists at all opioid receptors (e.g., morphine and hydromorphone), whereas others are partial agonists–antagonists (e.g., pentazocine, butorphanol, and nalbuphine) at the opioid receptors.
| Oral Dose Equianalgesic to Morphine 10 milligrams SC (milligrams) | Parenteral Dose Equianalgesic to Morphine 10 milligrams SC (milligrams) | Duration of Analgesic Action* (h) | Elimination Half-Life* (h) | |
|---|---|---|---|---|
| Opiate | ||||
| Codeine | 200 | 120 | 4–6 | 2.5–4 |
| Morphine | 30 | 10 | 3–4 | 2–4 |
| Semisynthetic | ||||
| Buprenorphine | 4 SL | 0.3 | 6–24 | 20–44 |
| Hydrocodone | 30 | Not available | 4–6 | 8 |
| Hydromorphone | 7.5 | 1.5 | 2–4 | 2–3 |
| Oxycodone | 20 | Not available | 3–6 | 3–4 |
| Oxymorphone | 6 | 1.5 | 4–6 | 7–11 |
| Synthetic | ||||
| Diphenoxylate | 2.5 | Not available | Not applicable | 2 h for diphenoxylate and 12–14 h for difenoxin† |
| Fentanyl | 0.125 | 0.100 | 1 | 3–4 |
| Levorphanol | 1 | 2 | 1–3 | 10–11 |
| Meperidine | 300 | 100 | 1–3 | 3–4 |
| Methadone | 20 | 10 | 4–8 | 12–18 |
| Pentazocine | 150 | 50 SC | 3–4 | 2–4 |
| Propoxyphene | 130 | Not available | 4–6 | 6–12 |
| Tapentadol | 75 | Not available | 4–6 | 4–5 |
| Tramadol | 100 | 100 | 4–6 | 5–7 |
Opioids are readily absorbed, achieving peak blood levels 30 to 60 minutes after ingestion of standard oral formulations. Sustained-release forms take longer after ingestion to achieve peak blood levels; for example, morphine sulfate controlled-release tablets take about 90 minutes to reach peak blood levels compared with 30 minutes for standard morphine tablets. After GI absorption, most opioids undergo first-pass hepatic metabolism, so bioavailability can vary from as low as 10% to as high as 80% after PO administration. Thus, at equal doses, most opioids are more potent given parenterally than PO. The opioids with good oral bioavailability are codeine, oxycodone, methadone, hydromorphone, and tapentadol.
The metabolism of codeine, meperidine, methadone, morphine, oxycodone, and propoxyphene is mostly hepatic and subject to drug interactions and genetic variations. For example, antiretroviral medications can enhance the metabolism of methadone, which results in lower plasma methadone concentrations. As another example, codeine is metabolized to morphine via cytochrome P-450 isoenzyme 2D6, an enzyme with genotypic and phenotypic variability. Patients with rapid cytochrome P-450 enzyme 2D6 metabolism produce more morphine after a fixed dose of codeine.4 These interactions and genetic variations may influence the therapeutic effect of opioids (see chapter 35, “Acute Pain Management”).
In drug misuse, unconventional routes of administration (insufflating or injecting ground opioid tablets, heating fentanyl patches or applying more than one patch to the skin) may alter the drug’s pharmacokinetics and often increase the rate of opioid absorption. Similarly, in cases of opioid overdose resulting in high plasma levels, the pharmacokinetics are altered due to enzymatic saturation. This may increase the severity of poisoning, delay the onset, and prolong the duration of action, as compared with the expected therapeutic actions.5
CLINICAL FEATURES
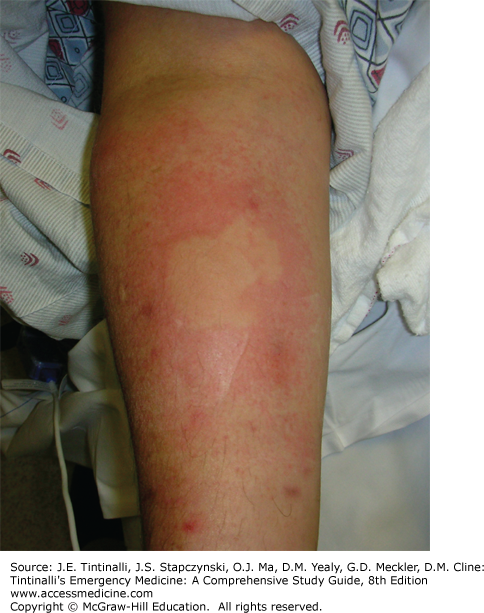
The full opioid intoxication toxidrome includes respiratory and mental status depression, analgesia, miosis, orthostatic hypotension, nausea and vomiting (especially in opioid-naïve patients), histamine release resulting in localized urticaria (Figure 186-1) and bronchospasm, ileus secondary to decreased GI motility, and urinary retention secondary to increased vesical sphincter tone.6 The depression in mental status can be profound. The respiratory depression is characterized by slow and shallow respirations that can produce hypercarbia, hypoxia, and cyanosis. Miosis is not universally present from intoxication with every opioid; normal or even enlarged pupils have been documented secondary to diphenoxylate, meperidine, morphine, pentazocine, and propoxyphene toxicity. Mydriasis may also signal severe cerebral hypoxia or result from co-ingestants. Other possible findings include pulmonary edema, hypothermia, rhabdomyolysis, compartment syndrome, myoglobinuric renal failure, and seizures associated with overdoses of tramadol, propoxyphene, and meperidine.
Opioid-induced acute lung injury, previously known as noncardiogenic pulmonary edema, is an uncommon complication associated with heroin overdose.6,7 Acute lung injury can occur immediately or be delayed up to 24 hours after heroin overdose and presents with tachypnea, rales, decreased oxygen saturation, and bilateral pulmonary infiltrates with a normal cardiac silhouette on chest radiograph. The incidence of acute lung injury is 10% in patients with severe heroin overdose requiring naloxone.8 The pathophysiology of heroin-induced acute lung injury is poorly understood, but some degree of direct capillary injury is suspected. Treatment includes oxygen supplementation and ventilatory support, either noninvasive or invasive modalities, and positive end-expiratory pressure. Additional naloxone, diuretics, and digoxin are not indicated.
The combination of meperidine, tramadol, or dextromethorphan with monoamine oxidase inhibitors, selective serotonin reuptake inhibitors, or linezolid can result in serotonin syndrome (see chapter 178, Atypical and Serotonergic Antidepressants).9 Serotonin syndrome is characterized by disorientation, hyperthermia, autonomic instability, hyperreflexia, and muscle rigidity, especially in the lower extremities. Although uncommon, deaths have been reported. Naloxone is not effective in treating opioid-induced serotonin syndrome.9,10
DIAGNOSIS
The combination of coma, miosis, and respiratory depression strongly suggests opioid intoxication, and in many clinical scenarios, evidence of opioid use is present. The combination of a respiratory rate of <12 breaths/min, miosis, and circumstantial evidence of opioid use (drug paraphernalia, needle marks, presence of a tourniquet, bystander corroboration) was highly sensitive for opioid overdose.11
Listen for auscultatory findings suggestive of pulmonary edema. Undress the patient completely and look for hidden opioids or drug-use paraphernalia, check for fentanyl patches on all parts of the body, including mucous cavities, and palpate muscle groups to detect signs of compartment syndrome.
The differential diagnosis of opioid intoxication includes toxicologic exposure to agents that produce similar findings, such as clonidine, organophosphates and carbamates, phenothiazines and atypical antipsychotic medications, sedative-hypnotic medications, and carbon monoxide. Clonidine overdoses are characterized by coma, bradycardia, hypotension, miosis, and periods of apnea that respond to tactile or auditory stimulation. Organophosphate and carbamate overdoses cause the cholinergic toxidrome: miosis, muscle fasciculations, profuse vomiting and diarrhea, and sweating. Phenothiazines, olanzapine, and risperidone cause neurologic depression and miosis from decreased adrenergic tone.12 γ-Hydroxybutyrate intoxication is associated with profound CNS depression, bradypnea, and, occasionally, miosis. Sedative-hypnotic agents and carbon monoxide cause profound neurologic depression but are not usually associated with miosis. Hypoglycemia, hypoxia, CNS infections, postictal states, and pontine and intracranial hemorrhages should also be considered in the differential diagnosis.
A qualitative urine opioid screen may aid in the diagnosis, but available tests have limitations. The assay in most commercially available urine opioid screens recognizes morphine.13 Therefore, morphine and those opioids that are metabolized to morphine, such as codeine and heroin, are readily detected. However, the semisynthetic opioids hydrocodone and oxycodone are usually not detected by urine opioid screens, and essentially all synthetic opioids also are not routinely detected. A specific urine assay is required to detect oxycodone or methadone.
Urine opioid screens can have false-positive results.13 Depending on the threshold level, ingestion of poppy seeds contained in baked goods can lead to a false-positive test result. Rifampin, rifampicin, quinine, diphenhydramine, and fluoroquinolones have been reported to cause false-positive urine opioid screen results, presumably by interfering with the immunoassay technique. An opioid analogue, dextromethorphan, can produce a positive result on the urine opioid screen. Prescription drugs reported to cause a false-positive result with the methadone urine drug screen include chlorpromazine, clomipramine, diphenhydramine, doxylamine, ibuprofen, quetiapine, thioridazine, and verapamil.
A urine opioid screen can be positive up to 2 to 3 days after a single use of codeine, morphine, or heroin. The methadone-specific screen can be positive up to 3 days after ingestion. Thus, detection of an opioid agent taken in the recent past may not correctly identify the toxicologic cause of the patient’s current condition. Plasma acetaminophen concentration should be obtained in all suicidal ingestions and all cases of combination opioid-acetaminophen overdoses.
TREATMENT
Airway protection and ventilatory maintenance are the most important treatment steps for opioid intoxications, because respiratory depression is the major morbidity and the cause of essentially all the mortality.6 Use bag-valve mask ventilatory support as needed to initially maintain adequate oxygenation and ventilation. After adequate ventilation is ensured, administer naloxone (Table 186-2). In fully awake patients or after the airway is protected with an endotracheal tube in unresponsive patients, administer single-dose activated charcoal, 1 gram/kg PO, if an opioid ingestion occurred within the hour. Endotracheal intubation is a therapeutic option in some cases of opioid overdose with severe respiratory depression unresponsive or poorly responsive to naloxone or in cases in which acute lung injury is suspected.6 Intubation offers the advantages of protection of the airway, easy access for suctioning, provision of an alternate route of administration for some medications, and total airway control. Rapid-sequence intubation, omitting anesthetic-sedative agents, is the preferred technique.
| Drug | Route | Initial Dose* | Onset of Action | Duration of Action† |
|---|---|---|---|---|
| Naloxone | IV IM or SC Intranasal Nebulized | 0.1–0.4 milligrams if breathing spontaneously 2 milligrams if apneic 2 milligrams 2 milligrams (1 milligram in each nostril) 2 milligrams in 3 mL normal saline | 1–2 min 5–6 min 6–8 min 5 min | 20–90 min |
| Nalmefene | IV IM or SC | 0.1–0.5 milligrams if breathing spontaneously 2 milligrams if apneic 1 milligram | 2–5 min 2–5 min 5–15 min |