CHAPTER 74
Occipital Nerve Stimulation
INTRODUCTION
Occipital neuralgia (ON) is a neurological disorder that commonly involves the greater occipital and less commonly the lesser occipital nerves.1 Trauma of the C2 root such as excessive movements (whiplash injuries) of the head and arthritic changes of the atlantoaxial joint are considered the most common causes of ON,1 but prolonged contraction or spasm of the posterior neck muscles from a variety of causes has also been implicated.1 The quality of the pain is classically similar to trigeminal and glossopharyngeal neuralgia but is localized in the occipital and periauricular areas, and may radiate into the retro-orbital area.2 As with neuropathic pain in other nerve distributions, a constant, nonlancinating pain is also common and may be termed as occipital neuropathic pain.
HISTORY
The concept of neuromodulation for the treatment of chronic intractable pain was developed almost 50 years ago.3 Although the Melzack-Wall gate control theory was generally accepted in the past, alternative mechanisms have been demonstrated to be responsible for the suppression of pain during neurostimulation such as4,5:
• Direct block of depolarization of the A-alpha, A-beta, and the A-delta fibers, as well as axonal conduction block are currently more accepted as the mechanisms of pain modulation from nerve stimulation.6
• Torebjork and Hallin demonstrated that repetitive stimulation of peripheral nerves results in excitation failure of C fibers thought to be responsible for conduction of painful stimuli.7
• Alternatively, peripheral nerve stimulation may block more distal nociceptive input by inhibitory action at the dorsal horn, brain stem, thalamus, or parietal cortex.8
The pathophysiological mechanisms responsible for pain control in ON with PNS are complex, and much remains to be learned. In 1999, Weiner and Reed reported percutaneous implantation of cylindrical electrodes in the vicinity of the occipital nerves for occipital neuralgia.9 Shortly thereafter, transformed migraines, that combine the features of both migraine and tension-type headache, were also treated with this technique.10 This relatively straightforward and less invasive approach quickly gained popularity and developed in terms of electrode type, insertion procedure, and indications.11 In 2003, Popeney and Alo postulated that the partial convergence of afferents from the occipital and frontotemporal region may account for a better clinical outcome with combined stimulation of the trigeminal and occipital nerves (ie, the trigeminocervical tract).12 This was later confirmed by others10–13 including Reed et al, who stimulated both the occipital and trigeminal systems in patients of refractory to ONS and ongoing holocephalic migraine.13 Their results support the need to evaluate both the C1-2-3 roots (occipital) and the supraorbital (trigeminal V1)/superficial temporal (trigeminal V3) nerves in refractory holocephalic headache.
INDICATIONS
PNS for the treatment of occipital neuralgia was described by Weiner9 in 1999 (Figure 74-1). Indications later expanded to include:
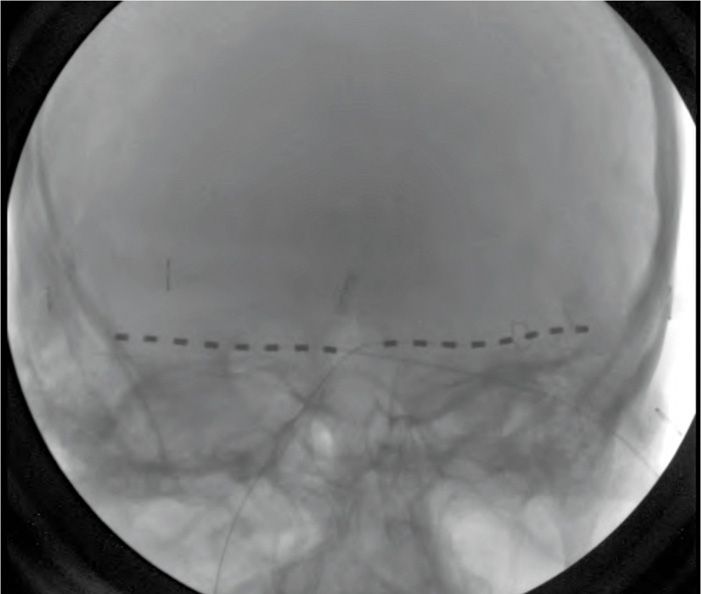
Figure 74-1 AP radiograph showing bilateral 8-contact occipital nerve stimulation electrodes.
• Cervicogenic headaches14
• Occipital neuroma15
• Craniofacial neuropathic pain16
• Migraines12
• Cluster headaches17
Some of these conditions have overlapping indications with trigeminal (eg, supraorbital) nerve stimulation. In general, indications for PNS include neuropathic pain syndromes with preserved sensation over the painful area.18 In the occipital region, the indications are the following:
• Post-traumatic or postsurgical neuropathic pain related to dysfunction of the occipital nerve
• “Transformed” migraine localized to the occipital area
• Cervicogenic occipital pain
The pain should have an anatomic distribution, be medically intractable, the patient must have favorable neuropsychological testing, and the painful area must not be anesthetic (hypesthesia or hyperesthesia are acceptable).18
CONTRAINDICATIONS
• Inadequately treated depression
• Somatization disorder
• Drug abuse
• Drug seeking behavior
• Secondary gains
ANATOMY OF THE GREATER AND LESSER OCCIPITAL NERVES
The occipital nerve provides sensory distribution to the posterior aspect of the scalp, the side of the neck, the shoulders, and the face.19 The 2 nerves primarily involved in the pathophysiology of ON are the greater occipital nerve (GNO) and lesser occipital nerve (LON).
• The GON is formed predominantly by the posterior ramus of C2; however, additional contributions from C1 and C3 roots have been described.1
• The LON is formed from the posterior primary rami of C2 and C3.1
• The roots have connections with the spinal accessory nerve and the superior sympathetic ganglion.19
• The nerves also receive branches from the greater auricular nerve and the trigeminal nerve.1
The GON and LON travel through the atlantoepistrophic ligament penetrating the trapezius, supraspinatus, scalenius, and temporalis muscle.1 The GON passes backward between the atlas and the axis in the space between the inferior oblique capitis and the semispinalis muscle.19 It pierces the semispinalis muscle and passes through a small aperture in the aponeurosis of the trapezius muscle, and finally emerges in the occiput.19 The nerves are relatively easy to target for stimulation, with palpable anatomic landmarks.
• Tenderness of the GON palpable over the occipital protuberance or just lateral to the insertion of the trapezius muscle into the occipital bone.19
• Tenderness of LON may be palpated about 3 cm superomedial to the tip of the mastoid process.19
Common injury sites, where GON is most vulnerable, are:
• Bony surfaces behind the lateral auricular masses of the atlas and axis, where it is not protected by pedicles or facets
• Where GON perforates the atlantoaxial membrane, especially during subluxation
• Where the nerve contacts the tendinous attachment of the trapezius to the suboccipital base
PREOPERATIVE CONSIDERATIONS
The following factors may affect surgical outcome2:
• Scar tissue. Scar formation from previous surgeries or trauma can make electrode placement more difficult.
• Integrity of the nerve. Deafferentation or discontinuity along the nerve may prevent effectiveness of stimulation. Concern must be especially high in patients with prior ablative procedures.
• Neuropsychological clearance. Severe depression, drug abuse, unrealistic expectations, a long history of multiple procedures, or possible secondary gains may predict unsatisfactory outcome.
• Diagnostic nerve blocks. Temporary relief of pain is one of the most important factors that predict favorable outcome.
Criteria Used to Assess the Results of Treatment
• Visual analogue scale (VAS) at baseline, during the trial, and after permanent implantation
• Medication intake before and after the implantation of the system
• SF-36 health survey, or other validated quality of life tool, to assess the patient’s physical and mental well-being
SURGICAL PROCEDURE
Anatomical Landmarks
• The anatomical location of the occipital nerves may vary from patient to patient.20
• The anatomical localization is best done with palpation of the GON 2 cm lateral and inferior to the external occipital protuberance.20
• Ultrasound is useful for visualizing the neurovascular structures directly.21
Trial Stimulation
Trial stimulation is commonly performed under local anesthesia and/or sedation. General anesthesia is often not used for the following reasons:
• To ensure adequate patient feedback and optimal coverage of the painful area using intraoperative stimulation.
• The trial procedure is short in duration.
• The occipital nerves are superficial, sedation supplemented with infiltration of the site with local anesthetic will typically suffice.
Patient Positioning
The patient is positioned prone on the operating table with the head midline and slightly flexed to expose the occipital surface, with support under the chest and forehead, and prepared and draped in sterile fashion over the occipital area and neck.
Fluoroscopic Views
The proper placement of electrodes is usually verified with anteroposterior fluoroscopy. Alternatively, ultrasound can be used for accurate real-time localization of nervous and vascular structures.21 The ultrasound probe is inserted at the midline just below the external occipital protuberance and slowly advanced laterally (Figures 74-2 to 74-5).
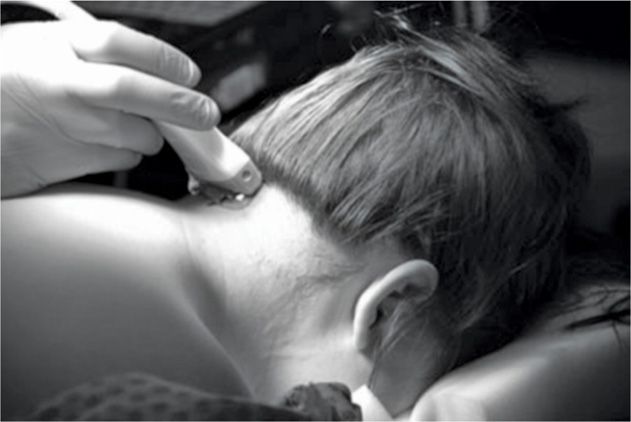
Figure 74-2 The ultrasound probe placed in the midline just below the external occipital protuberance. (Reproduced with permission from © 2010 International Neuromodulation Society Skaribas, I. and Aló, K. (2010), Ultrasound imaging and occipital nerve stimulation. Neuromodulation: Technology at the Neural Interface, 13: 126-130. doi: 10.1111/j.1525-1403.2009.00254.x.)
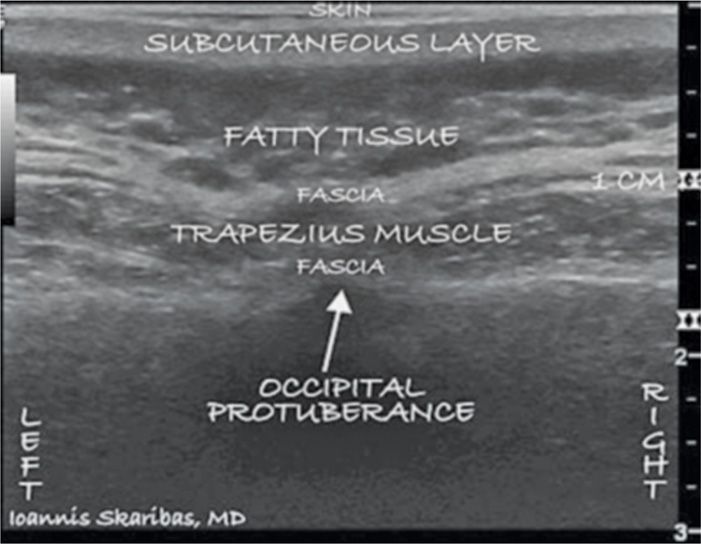
Figure 74-3 Sonographic image of the external occipital protuberance and bilateral occipital fossae. (Reproduced with permission from © 2010 International Neuromodulation Society Skaribas, I. and Aló, K. (2010), Ultrasound imaging and occipital nerve stimulation. Neuromodulation: Technology at the Neural Interface, 13: 126-130. doi: 10.1111/j.1525-1403.2009.00254.x.)
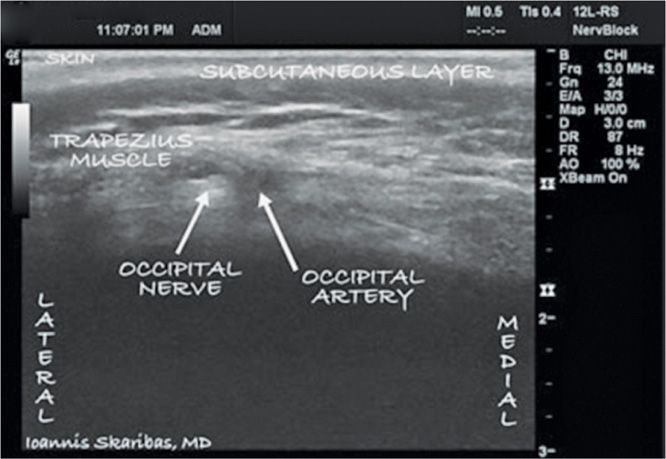
Figure 74-4 The ultrasound probe was first placed in the midline below the external occipital protuberance. Then it was slowly advanced laterally at the same level until images of the greater occipital artery and nerve were obtained as 2 distinct structures: the artery as a hypoechogenic oval structure located medially and the nerve as a hyperechogenic structure located laterally. (Reproduced with permission from © 2010 International Neuromodulation Society Skaribas, I. and Aló, K. (2010), Ultrasound imaging and occipital nerve stimulation. Neuromodulation: Technology at the Neural Interface, 13: 126-130. doi: 10.1111/j.1525-1403.2009.00254.x.)
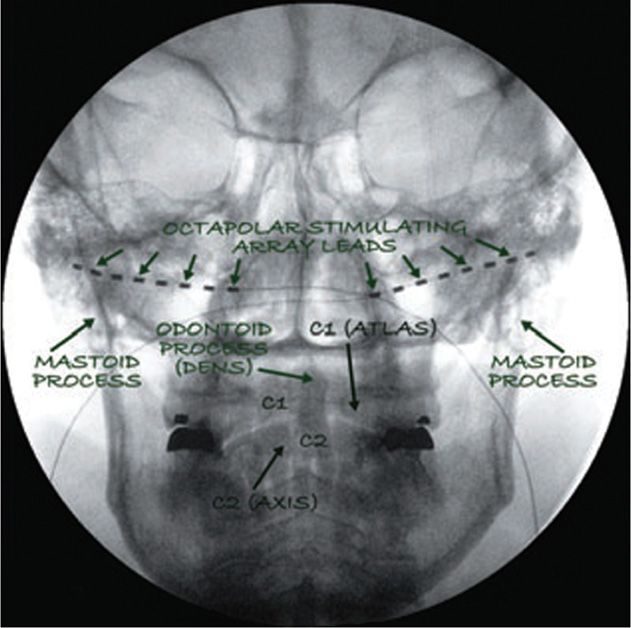
Figure 74-5 Two 8 contact stimulating leads (octrodes, Saint Jude Medical, Plano, TX, USA) as they are positioned bilaterally after ultrasound-guided placement. This fluoroscopic picture confirms the higher lead placement (note the level of C1-C2 compared with the level of the leads). (Reproduced with permission from © 2010 International Neuromodulation Society Skaribas, I. and Aló, K. (2010), Ultrasound imaging and occipital nerve stimulation. Neuromodulation: Technology at the Neural Interface, 13: 126-130. doi: 10.1111/j.1525-1403.2009.00254.x.)
Intraoperative Steps
• The landmarks for placement of occipital nerve stimulation electrodes are C1-C2 interspace, the occipital protuberance and the mastoid processes.
• The goal is that the electrode should cross the targeted nerve about 5 mm superficial to it. Both the occipital artery and greater occipital nerve can be identified 1 to 1.5 cm below the skin.
• The stimulator is subsequently introduced 0.5 to 0.7 cm below the skin surface.
• Confirm the optimal positioning of the needle under fluoroscopic guidance.
• A 4-contact Quattrode electrode is inserted through the needle in such a way that all 4 contacts stay in close proximity to the greater occipital nerve.
• The appropriate position may or may not be verified with intraoperative test stimulation of the awake patient.
• For a good long-term result, radiating paresthesia must be obtained within the distribution of the C1-2-3 roots (occipital nerves).
• Results may be limited if a localized grabbing or nonradiating paresthesia is generated. Experienced practitioner may not require routine intraoperative stimulation due to the relatively reliable superficial distribution of the greater occipital nerve.
• Some practitioners do not perform intraoperative stimulation and rely exclusively on the anatomical positioning of electrode in proximity of the greater occipital nerve.
• After proper positioning, the electrode is draped in a sterile fashion and connected to the external stimulation device.
• The patient is discharged home on the same day.
If the trial is successful (pain reduction of >50%), then the patient undergoes permanent implantation of the system 1 to 1 weeks after the trial electrodes are removed in the office.
Permanent Implantation
Permanent implantation is performed under general anesthesia. An occipital incision is made, and the anatomy of the successful trial recreated. A subcutaneous pocket is formed to allow for placement of the internal pulse generator (IPG), typically in the subclavian area or on the back. An adequate pocket should meet the following criteria18:
• It must be deep enough to avoid erosion
• Too deep of an insertion may interfere with programming or charging. This is dependent on the specific model of device—consult the manufacturer’s recommendations.
• The insertion site should be in a relatively immobile location to avoid potential electrode or extension failure due to mechanical stresses.
Tunneling involves creation of an incision and tissue dissection down to the fascia. The extension cable is passed through a subcutaneous tunnel to the pocket site to establish a connection with the IPG.
Postprocedure Considerations
The stimulator is turned on when the patient is awakened from the procedure to ensure adequate coverage. Some centers allow up to several weeks before turning the device on. A comprehensive programming session is important to ensure the most optimal coverage of painful areas. The stimulation parameters vary from patient to patient; therefore, programming is performed on an individual basis. The surgeon should follow their own routine protocol for wound checks and follow up, keeping in mind that the patients may require additional visits for programming adjustments initially.
MONITORING AND POTENTIAL COMPLICATIONS
Complications can be divided into surgical complications and hardware complications.
Surgical complications:
• Infection
• Wound hematoma
• Seroma
Monitor for hardware related complications:
• Lead migration
• Lead failure
• Pulse generator failure
CLINICAL PEARLS AND PITFALLS
• The trajectory to cover the occipital nerves is based on palpable landmarks. If you span that region with electrodes, the relevant nerves are usually covered.
• Ultrasound can clarify the primary concern of depth to generate adequate radiating paresthesia.
• Migration is a major consideration. Give adequate thought to anchoring and relaxation loops in the planning and positioning.
• Headaches are a heterogeneous treatment group. Specifically assess the contribution of other structures, especially the trigeminal nerves (supraorbital V1 or superficial temporal V3) before instituting treatment of the occipital nerves alone.10,12
• The use of any implantable stimulator system for cranial peripheral nerve treatment is off-label with the FDA and this should be mentioned during the informed consent process.
• The radiofrequency system from St. Jude Medical is currently approved for peripheral nerve stimulation and thus indicated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







