Chapter 80
Obesity Hypoventilation Syndrome and Other Sleep-Related Breathing Disorders
Obesity hypoventilation syndrome (OHS) falls on the severe end of the spectrum of sleep-related breathing disorders (SRBDs) (Table 80.1). Because obesity is a leading risk factor for SRBDs, as the prevalence of obesity and morbid obesity increases, patients with SRBDs, including obstructive sleep apnea (OSA) and OHS, are increasingly admitted to intensive care units (ICUs).
TABLE 80.1
Spectrum of Sleep-Related Breathing Disorders
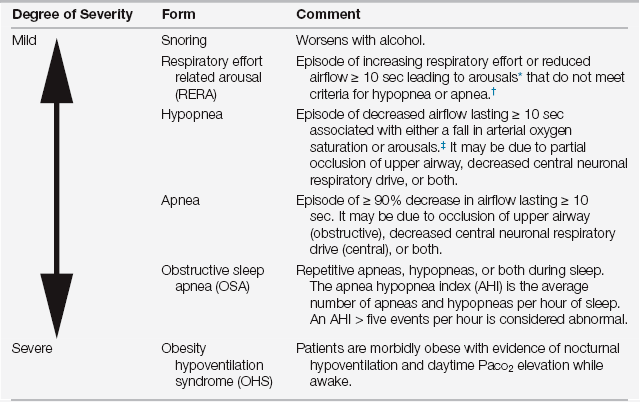
∗Arousals: an electroencephalographically defined change in state from a deeper stage of sleep to a lighter stage of sleep or to wakefulness, often manifested by the presence of alpha activity.
†≥ 5 RERAs per hour and daytime hypersomnolence was previously called upper airway resistance syndrome (UARS).
‡Variable definitions of hypopnea exist that differ in the degree of airflow reduction (30% to 50%) and oxyhemoglobin desaturation (3% to 4%).
It is not fully understood why some patients move along this continuum to develop the more severe SRBDs and hypercapnia and some with the same rate of weight gain or degree of obesity do not. Nonetheless, physicians need to be aware of the physiologic derangements and ICU complications associated with morbid obesity (Chapter 29), including SRBDs such as OHS.
Obstructive Sleep Apneas (OSAs)
Clinical features suggestive of OSA include snoring, snorting or gasping, witnessed apnea, and daytime somnolence. Patients typically have large neck circumference measurements (men > 17 inches, woman > 15 inches), macroglossia, elongated or enlarged soft palate, lateral narrowing of the pharynx, tonsillar hypertrophy, retrognathia, and a crowded upper airway (e.g., class 4 modified Mallampati class; Figure 80.1).
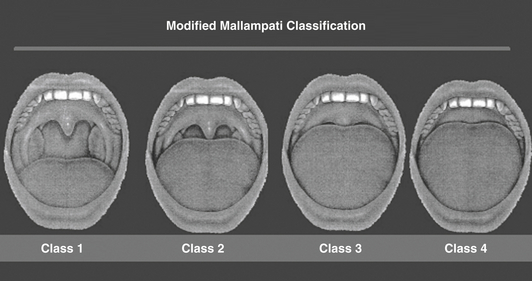
Figure 80.1 Modified Mallampati classification.
The patient’s oral pharynx is examined in the sitting position with the tongue protruded voluntarily. For class 1, one can see the entire uvula and tonsils; for class 2, one can see the uvula but not the tonsils; for class 3, one can see the proximal soft palate but not the uvula; for class 4, one can see only the hard palate. Most patients with sleep apnea are class 3 or 4. (From Mallampati SR, Gatt SP, Gugino LD, et al: A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J 32:429-434, 1985.)
OSA is a systemic disorder associated with various clinical consequences including systemic hypertension, cerebrovascular disease, arrhythmias, coronary artery disease, congestive heart failure, mild pulmonary hypertension, and insulin resistance. Although patients may report loud snoring, apneas or choking witnessed by bed partners, excessive daytime sleepiness, unrefreshing sleep, nocturia, intellectual impairment, and irritability, a detailed sleep history is often limited in the ICU and a high index of clinical suspicion is necessary. This is particularly true in the obese patient, as obesity is the major risk factor for OSA. Oropharyngeal examination may be helpful in identifying ICU patients with obstructive sleep apnea. Such patients may have a high Mallampati classification—that is, class 3 or 4 during oral cavity examination (see Figure 80.1)—retrognathia, and enlargement of the tongue, lateral peritonsillar tissue, soft palate, and tonsils.
Central Sleep Apneas (CSAs)
Central sleep apnea (CSA) is characterized by repeated episodes of apnea during sleep in the absence of any respiratory effort. The pathogenesis of central sleep apnea is related to a transient loss of central nervous system drive to the respiratory muscles (Chapter 1). In contrast, in OSA, respiratory effort is preserved but airflow is prevented by upper airway occlusion. An overnight polysomnography (which monitors the electroencephalogram, extraocular muscles, airflow at mouth and nose, and movements of the chest wall, abdomen, and extremities) can differentiate central from obstructive apnea, but currently their utility in the ICU remains unknown. CSA, but not OSA, produces the absence of nasal-oral airflow and thoracoabdominal excursion observed on the polysomnogram.
Cheyne-Stokes respiration (CSR), a subtype of CSA, represents periodic breathing with alternations between apnea and hyperpnea (Figure 80.2). CSR manifests in patients with severe congestive heart failure (CHF) and stroke, with a cited prevalence in CHF varying widely between 30% and 100%. The primary hypothesized pathophysiology is hyperventilation, producing hypocapnia below the threshold required to trigger breathing.
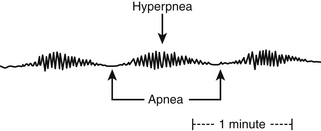
Figure 80.2 Cheyne-Stokes respiration.
The Cheyne-Stokes pattern of breathing is characterized by alternating periods of hyperpnea and apnea in a crescendo-decrescendo pattern. Typically, the duration of hyperpnea is longer than that of apneas.
However, CPAP, bi-level ventilation (bi-level), and adaptive servo-ventilation (ASV) complement medical therapy. ASV provides servo-controlled, independently varying expiratory and inspiratory pressure support, based on the detection of CSR with a backup respiratory rate. The machine servo-controls the patient’s ventilation to achieve a target of 90% of the long-term average ventilation. Studies utilizing ASV in CSR-CSA patients have reduced central events, micro-arousals, daytime sleepiness, and improved compliance compared to patients on CPAP or sham ASV. However, to date, no impact of ASV upon cardiovascular outcomes has been proven.
Obesity Hypoventilation Syndrome (OHS)
The definition of OHS is challenging and hampered by differing nomenclature and criteria. The American Academy of Sleep Medicine developed criteria for sleep hypoventilation syndrome, which subsumes OHS, although current literature commonly utilizes the latter term (Table 80.2). In spite of the discrepant definitions and criteria, the well-established phenotype of OHS includes obesity (especially a body mass index [BMI] > 40 kg/m2 that the CDC defines as morbid obesity) and chronic alveolar hypercapnia (daytime Pco2 > 45 mm Hg) in the absence of other etiologies of alveolar hypoventilation (such as existing pulmonary and neuromuscular disease). Oxyhemoglobin desaturations detected by nocturnal pulse oximetry can clue the diagnosis in an obese patient (Figure 80.3).
TABLE 80.2
Diagnostic Criteria for Sleep Hypoventilation Syndrome
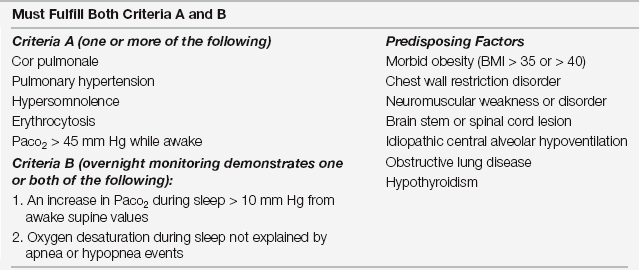
Adapted from Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 22:667-689,1999.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



