Chapter 15
Nutritional Therapy 
Nutritional and Metabolic Assessment
When Is the Best Time to Start?
Body Weight
Although seemingly a straightforward measurement, body weights in ICU patients are often distorted by efforts at volume resuscitation and changing fluid distribution among various body compartments. Consequently, the patient’s current weight should be compared with his or her “usual” weight as well as predicted body weight (PBW) (see formulas for PBW in Table 15.1), also referred to as lean or ideal body weight, with attention paid to estimating the “dry” weight of volume-overloaded patients.
TABLE 15.1
Caloric Prescriptions Based on Body Weight and Status of Fat Stores
| Current Body Weight Versus Predicted Body Weight∗ | Inferred Status of Fat Stores | Caloric Prescription |
| CBW < 90% PBW Or BMI < 18.5 kg/m2 | Subnormal | One should use sufficient energy supply to replete depleted fat stores (daily caloric supply > REE) |
| CBW 90%–130% PBW Or BMI 18.5–29.9 kg/m2 | Normal | The caloric prescription is designed to maintain the fat stores (daily energy supply = REE) |
| CBW >130% PBW Or BMI ≥ 30 kg/m2 | Excessive | Energy supply should be designed to partially utilize the excess fat stores; to do this, supply ~50% REE as nonprotein calories and 2 g protein/kg/day, assuming normal renal and hepatic function |
∗CBW is the best estimate of the patient’s current “dry” body weight (e.g., before volume resuscitation); predicted (or lean or ideal) body weight (PBW in kg) for males = 50.0 + 2.3 for each inch above 60 in height and for females = 45.5 + 2.3 for each inch above 60 in height.
Significant weight loss (> 10%) during the 6 to 12 months before the critical illness episode should signify a patient at high risk for clinically significant malnutrition. Despite its limitations, current weight, when compared with predicted body weight, can be used to estimate how the patient’s fat calorie stores compare with normal and can guide the appropriate caloric prescription.
Caloric Goal Delineation
Delineation of realistic clinical goals is important in order to prescribe appropriate cost-conscious prescriptions for total parenteral nutrition (TPN) or total enteral nutrition (TEN). To make a rational caloric prescription, one needs to know the individual patient’s total energy expenditure (TEE) as well as the patient’s caloric tissue goals. TEE defines the severity of hypermetabolism. TEE can be estimated by indirect calorimetry in many ICU patients but not those on ventilators with high inspired oxygen concentrations (FiO2 > 0.6) and positive end-expiratory pressure (PEEP) of 7.5 to 10 cm H2O since the measurement depends on the patient being able to tolerate a brief interruption of ventilation. In the ICU patient on bed rest, resting energy expenditure (REE) measured over 30 minutes generally approximates TEE. After determining TEE, the caloric (or “nonprotein” energy) prescription is guided by the clinician’s goal for the patient’s fat stores (Table 15.1).
If indirect calorimetry is not feasible, REE can be estimated by using the Harris Benedict or the Penn State equations with “multipliers” to take into account the hypermetabolic effects of critical illness (see online Table 15.E1). ![]() Some intensivists use an even simpler “one-size-fits-all” rule, such as providing 25 kcal/kg PBW. Even with the adjustments, these rules can still under- or overestimate actual TEE in hypermetabolic states, emphasizing the desirability of indirect calorimetry measurements if possible and the need for regular monitoring of the patient’s response to nutritional therapy as discussed further on.
Some intensivists use an even simpler “one-size-fits-all” rule, such as providing 25 kcal/kg PBW. Even with the adjustments, these rules can still under- or overestimate actual TEE in hypermetabolic states, emphasizing the desirability of indirect calorimetry measurements if possible and the need for regular monitoring of the patient’s response to nutritional therapy as discussed further on.
Protein Goal Delineation
During critical illness, protein is mobilized from many body tissues, such as the intestinal tract, skeletal muscle, albumin mass, and the skin. This provides precursors for crucial protein synthesis (e.g., acute-phase plasma proteins and immunoglobulins), wound healing, and for energy if other substrates are not readily available. The magnitude of this mobilization and redistribution can be impressive, with urinary nitrogen losses of 30 to 50 g/day typically observed in patients with multiple trauma or severe sepsis or after bone marrow transplantation. This represents a loss of greater than 1 kg of lean tissue each day (with loss of 30 g of lean tissue being roughly equivalent to loss of 1 g of nitrogen). Endogenous production of glutamine from skeletal muscle may become insufficient, making this generally nonessential amino acid conditionally essential.
Determining how much protein to provide by nutritional therapy should, in general, be independent of one’s total (nonprotein) caloric prescription (described earlier). How much protein to give to a critically ill ICU patient initially remains more or less an empirical decision. One acceptable approach for these circumstances is to give 2 g of protein/kg current body weight (assuming normal functioning hepatic and renal disposal systems [Table 15.2]). For sick but not critically ill ICU patients, daily protein administration on the order of 1.5 g/kg/day would be an appropriate starting point. In comparison, recommended daily protein intake for healthy, well-nourished adults is only on the order of 0.8 to 1 g/kg/day.
TABLE 15.2
Adjustments to Nutrition Prescription with Organ Dysfunction
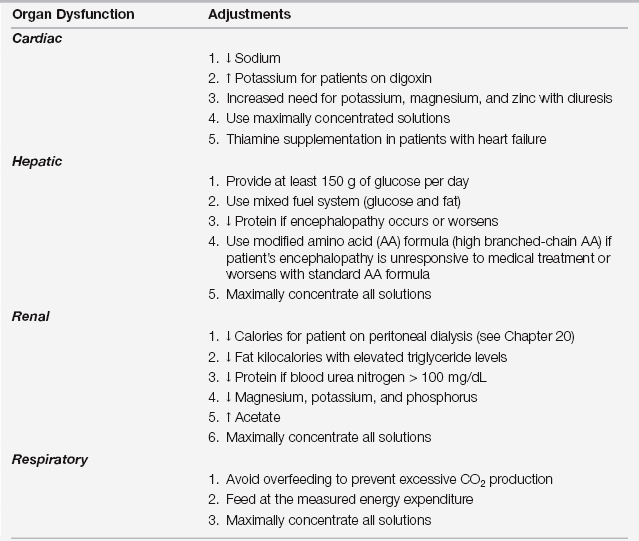
Adjustments to standard formulas: arrow up, increase; arrow down, decrease.
where 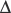 BUN (in g) = [0.6 x weight(f) x BUN(f)] – [0.6 x weight(i) x BUN(i)], BUN(i) and BUN(f) are the initial and final values of blood urea nitrogen (BUN expressed in g/L–not the laboratory output of mg/dL) and weight(i) and weight(f) are the initial and final weights (in kg) during the measurement period, respectively. Urinary nitrogen (in g) is measured as the concentration of urea in urine multiplied by the volume of a 24-hour urine collection.
BUN (in g) = [0.6 x weight(f) x BUN(f)] – [0.6 x weight(i) x BUN(i)], BUN(i) and BUN(f) are the initial and final values of blood urea nitrogen (BUN expressed in g/L–not the laboratory output of mg/dL) and weight(i) and weight(f) are the initial and final weights (in kg) during the measurement period, respectively. Urinary nitrogen (in g) is measured as the concentration of urea in urine multiplied by the volume of a 24-hour urine collection.
TABLE 15.E1
Estimates for Daily Caloric and Protein Needs
Data from Long CL, Schaffel N, Geiger JW, et al: Metabolic response to injury and illness: estimation of energy and protein needs from indirect calorimetry and nitrogen balance. JPEN 3:452-457, 1979; Gottschlich MM, Matarese L, Shonts E (eds): Nutrition Support Core Curriculum. Silver Spring, MD: American Society for Parenteral and Enteral Nutrition (ASPEN) Publishers, 1992; and Williamson J: Actual burn nutrition care practices: a national survey (Part II). J Burn Care Rehab 10:185-941, 1989.
Providing Nutritional Support
Selecting the Route of Administration
After determining that nutritional support is required, defining its goals, and writing the calorie and protein prescriptions for the patient, one must select the most appropriate route of administration. This decision, however, should not be dictated simply by what access the patient currently has available.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




