Chapter 15 Normothermia Management
Prevention of Harm From Perioperative Hypothermia
Perioperative temperature management is an important and proactive patient safety initiative because it may have tremendous impact on surgical patient outcomes, especially in high-risk patients. Over the past few decades, research on temperature homeostasis has illuminated a wide range of adverse pathophysiologic effects of perioperative hypothermia on surgical patients. Hypothermia, defined as a core body temperature below 36.0° C or 96.8° F, is a potentially harmful event that warrants assessment and intervention during each phase of perioperative care (Association of periOperative Registered Nurses [AORN], 2009). Box 15-1 lists the known consequences of hypothermia.
By a combination of behavioral and physiologic responses, the human body normally maintains a steady internal core temperature close to 37° C (98.6° F) despite significant environmental temperature changes. When a patient undergoes general anesthesia for longer than 30 minutes or a major procedure under neuraxial anesthesia (e.g., spinal or epidural anesthesia) for longer than 1 hour, hypothermia is to be expected. All general anesthetics produce a dose-dependent decrease in core temperature because of the disruption of both behavioral and physiologic mechanisms of thermoregulation. Neuraxial anesthesia approaches also impair temperature regulation, although to a lesser degree than does general anesthesia (Sessler, 2008).
Perioperative hypothermia occurs in either a planned or unplanned manner. Therapeutic hypothermia, a planned temperature management event, is used during surgeries that have tissue hypoxia and ischemia risks such as in cardiac surgery, neurosurgery, and organ transplantation; the maintenance of hypothermia lowers basal metabolic rates and oxygen consumption effects. Planned hypothermia is also used to reduce the risk for malignant hyperthermia development in potential surgical candidates. In planned hypothermia the rewarming of the patient is also planned and monitored (Kumar et al, 2005; Sessler, 2008). Conversely, unplanned hypothermia results from anesthesia-induced thermoregulation impairment, heat loss inherent during surgery, and the cold perioperative environment. As the duration of anesthesia time increases, the risk for unplanned hypothermia increases for all patients. These risk factors associated with the development of unplanned perioperative hypothermia are provided in Box 15-2. Unfortunately, unplanned hypothermia remains a patient safety problem because the use of routine prevention measures is not the standard of care for all patients undergoing surgery.
BOX 15-2 Risk Factors Associated With Perioperative Hypothermia
The maintenance of a normal core body temperature, or normothermia, is a persistent challenge for the perioperative team. Research evidence supports that normothermia maintenance is an effective way to avoid and/or treat many of the complications that occur with unplanned perioperative hypothermia (Wagner, 2006; AORN, 2009). This chapter will review both the physiology of temperature regulation and the etiology of unplanned perioperative hypothermia, the relationship between surgical complications and hypothermia, as well as approaches to prevent and treat unplanned hypothermia. Therapeutic hypothermia is briefly discussed to clarify the similarities and differences between the two types of hypothermia.
PHYSIOLOGY OF TEMPERATURE REGULATION
Human body temperature is a tightly controlled physiologic parameter that is normally controlled within 0.2 ° C (0.4 ° F). The normal core temperature ranges from 36 ° C to 38 ° C (96.8 ° F to 100.4 ° F), with every individual recognized as having a unique baseline core temperature within this range. Values less than 36 ° C (96.8 ° F) or greater than 38 ° C (100.4 ° F) usually indicate a loss of thermoregulatory control or an extreme thermal environment that overwhelms thermoregulatory defenses (Sessler, 2008). Even small variations in the core body temperature stimulate antagonistic thermoregulatory defenses, because maintenance of normothermia is necessary for life.
Thermoregulatory thresholds and control are similar in men and women, but notably decline in older adults. Thermoregulatory control is intact in slightly premature infants, but is presumed immature in the less-developed infant, such as those weighing less than a kilogram (Mestyan et al, 1964). Thresholds vary daily by 0.5 ° C to 1 ° C (0.9 ° F to 1.8 ° F) in both sexes because of circadian rhythm and by approximately 0.5 ° C (0.9 ° F) with menstrual cycles in women. Infection, thyroid disease, drugs (including sedatives, nicotine, and alcohol), exercise, nutrition, and thermal adaptation will all alter threshold temperatures; however, these alterations are small compared with the profound impairment that general anesthesia creates (Tayefeh et al, 1998; Sessler, 2008).
The major autonomic thermoregulatory mechanisms in humans are vasodilation, vasoconstriction, shivering, and sweating. The hypothalamus, which is the controlling center for the autonomic nervous system, acts as a thermostat to maintain body temperature within the narrow physiologic range of only 0.2 ° C to 0.4 ° C (0.4 ° F to 0.7 ° F). Vasodilation and sweating are mechanisms of heat dissipation, whereas vasoconstriction and shivering are the main heat-conserving mechanisms. Each of these defenses has a threshold or set point that involves an incremental temperature change due to response as a maximum intensity response. Sweating and vasoconstriction thresholds are separated only by a few tenths of a degree Celsius, whereas the shivering threshold is a full degree Celsius below the vasoconstriction threshold. This interthreshold range defines the normal range of body temperature (Sessler, 2008).
Thermoregulation is the balance between heat loss and heat gain, which determines core temperature of the human body. The thermoregulatory system consists of a sensory component, a control center, and effector mechanisms. The hypothalamus control center maintains normothermia by balancing heat production, heat conservation, and heat loss hormonally. Peripheral thermoreceptors in the skin and central thermoreceptors in the hypothalamus, spinal cord, abdominal organs, and other central locations provide the hypothalamus with regulatory information about skin and core temperatures. If skin and core temperatures are low, the hypothalamus responds by triggering heat-production and heat-conservation mechanisms. Increased heat production is initiated by a series of hormonal mechanisms involving the hypothalamus and its connections with the endocrine system. The heat-producing mechanism begins with a hypothalamic hormone, thyrotropin-stimulating hormone–releasing hormone (TSH-RH). TSH-RH in turn stimulates the anterior pituitary to release thyroid-stimulating hormone, which acts on the thyroid gland, stimulating release of thyroxine, one of the thyroid hormones. Thyroxine then acts on the adrenal medulla, causing the release of epinephrine into the bloodstream. Epinephrine causes vasoconstriction, stimulates glycolysis, and increases metabolic rates, thus increasing heat production (Silva, 2005).
The hypothalamus also triggers heat conservation. The mechanisms of heat conservation involve stimulating the sympathetic nervous system, which is responsible for stimulating the adrenal cortex, increasing skeletal muscle tone, initiating the shivering response, and producing vasoconstriction. The hypothalamus also functions in raising body temperature by relaying information to the cerebral cortex (Silva, 2005). Awareness of cold provokes voluntary responses such as increased body movement. Behavioral responses require a conscious perception of body temperature. Minute changes in skin surface temperature are easily perceived; however, changes in central temperature are poorly sensed by humans. In fact, behavioral thermoregulation is half mediated by skin temperature, whereas the actual mean skin temperature contributes only 10% to 20% to the autonomic control of thermoregulatory defenses (Frank et al, 1999).
The body can also be described as two thermal compartments: a core compartment and a peripheral compartment. The core compartment houses the central nervous system and the organs in the skull, chest, and abdominal cavity. The peripheral compartment surrounds the core and includes the skin, fat, and muscles—tissues in which temperature is nonhomogeneous. A chief characteristic of the peripheral compartment is that heat content and distribution change significantly over time and as a function of environmental exposure. This is in striking contrast to the core compartment, for which temperature is usually precisely regulated. The peripheral compartment temperature ranges from 31 ° C to 35 ° C (87.8 ° F to 95 ° F), with skin temperature at 28 ° C to 32 ° C (82.4 ° F to 89.6 ° F). The core compartment is kept constant at about 37 ° C (98.6 ° F), irrespective of the environmental temperature. The peripheral compartment functions as a thermal buffer—either absorbing or releasing heat to the environment—protecting the core compartment by maintaining a constant temperature for the vital organs. This peripheral thermal buffer is largely controlled by arteriovenous shunts located in the extremities (Sessler, 2008).
The skin of the upper portion of the chest, as well as the skin on the face, is the most sensitive to temperature. The widespread belief that much of the body heat is lost through the head is untrue (Sessler, 2008). Remember, the head encases the brain and is part of the core compartment.
PERIOPERATIVE HYPOTHERMIA RISK FACTORS
General Anesthesia
General anesthesia obliterates behavioral adaptive responses and impairs vasoconstriction, and direct peripheral vasodilation effects cause the patient’s core temperature to drop up to 1.6 ° C (2.7 ° F) during the first hour of anesthesia. This drop in core temperature can be explained by redistribution of heat from the body’s core to the periphery. Redistribution occurs because anesthetics inhibit thermoregulatory control and disrupt the tonic vasoconstriction that normally maintains a core-to-peripheral temperature gradient. Anesthesia agents decrease vasoconstriction thresholds by 2 ° C to 4 ° C (3.6 ° F to 7.2 ° F) with the opening of arteriovenous shunts (Matsukawa et al, 1995; Sessler, 2008). Figure 15-1 depicts the decrease in body temperature that occurs when heat is redistributed from the body’s core compartment to the peripheral tissues. Not a clear exchange of heat with the environment, this redistribution is a heat flow from the actual core to the periphery, resulting in decreased core temperatures. Anesthetics inhibit thermoregulation in a dose-dependent manner and inhibit vasoconstriction and shivering approximately three times more than they inhibit sweating (Sessler, 2008).
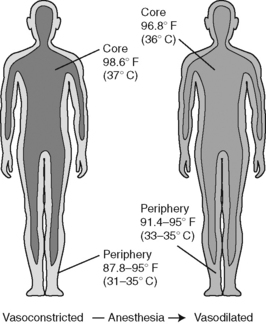
Figure 15-1 Core-to-peripheral redistribution of heat after anesthesia administration.
(From Sessler DI: Perioperative heat balance, Anesthesiology 92:583, 2000.)
The next phase of the hypothermic action is a slower, linear decrease in core temperature with heat loss exceeding heat production. This phase lasts approximately 2 to 3 hours and depends on the difference between loss and metabolic heat production. During this time, core temperature continues to decrease an additional 1.1 ° C (2 ° F). Approximately 90% of heat loss is through the skin surface, with convection and radiation usually contributing more to the process than evaporation or conduction. Heat from the patient is transferred to the environment in four ways: radiation, convection, conduction, and evaporation (Figure 15-2) (Kurz et al, 1995). Box 15-3 lists heat loss mechanisms that occur in the perioperative setting. If patients are actively warmed during this phase, the heat loss can be effectively limited.
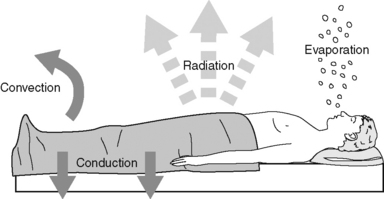
Figure 15-2 Mechanisms of heat loss.
(From Sessler DI: Perioperative heat balance, Anesthesiology 92:583, 2000.)
BOX 15-3 Heat Loss Mechanisms
CONVECTION
After 3 to 5 hours of anesthesia, there is a plateau phase that may reflect a steady state of heat loss equaling heat production and is seen in patients who are well insulated. However, if a patient is quite hypothermic, an arrested temperature decline results from activation of thermoregulatory vasoconstriction, which decreases cutaneous heat loss and acts to hold metabolic heat in the body core. Typically this happens when the patient’s core temperature is about 34 ° C (93.2 ° F) (Sessler, 2008).
Regional Anesthesia
Regional anesthesia decreases the vasoconstriction and shivering to a slighter loss, approximately 0.6 ° C (1 ° F), and is dependent on the level of the block. Although the magnitude of response is less, the pattern of thermal impairment is similar to that of general anesthetics. This similar pattern of impairment suggests an alteration in central rather than peripheral control of temperature (Heier and Caldwell, 2006; Sessler, 2008). Core hypothermia during regional anesthesia may not be recognized by the patient. The reason is that thermal perception and behavioral regulation are mainly determined by skin rather than core temperatures. During regional anesthesia, skin temperature may increase even when there is core hypothermia present. The patient often has the perception of warmth, but will have autonomic thermoregulatory responses, including shivering (Sessler, 2008).
Sedatives and analgesics also impair thermoregulatory control to some degree (Sato et al, 2009). If combined with the impairment of regional anesthesia and other factors, such as a preexisting illness, the thermoregulatory impairment would be severe (Sessler, 2008).
Other Risk Factors
Besides anesthesia agents, there are numerous factors that place surgical patients at a higher risk for the development of unplanned hypothermia during surgery. To see a compilation of the risk factors, see Box 15-2. The ambient temperature of the operating room environment determines the rate at which metabolic heat is lost from the skin through radiation, convection, and evaporation. Skin preparation methods may also contribute to hypothermia through evaporative loss (Sessler et al, 1993). There is a greater risk for hypothermia during procedures in which large body surface areas are left exposed, in situations where the peritoneal cavity is opened, and during longer surgical procedures (Roe, 1971). Infusions of cool fluids, blood, and blood products have been shown to increase risk for hypothermia. A unit of refrigerated blood decreases mean body temperature approximately 0.25 ° C (0.45 ° F) in a 70-kg (154-lb) patient (Sessler et al, 1993; Camus et al, 1996; Hasankhani et al, 2007). Use of a pneumatic tourniquet helps prevent hypothermia while inflated; however, when deflated, an abrupt hypothermia occurs. Upon release of the cuff pressure, a redistribution of heat from the core compartment to the peripheral compartment results in a rapid decline in core temperature (Sanders et al, 1996; Akata et al, 1998). The use of cool irrigation solutions into the abdomen, pelvis, or chest cavity enhances heat transfer from the core and decreases body temperature with the increased heat loss (Moore et al, 1997).
Numerous clinical studies have found that hypothermia increases the risk for death in trauma patients. The trauma patient has predisposing factors from injuries and preexisting hypothermia from exposure in the field, blood loss and shock, rapid infusions of cool fluids, removal of clothing, and impaired heat production. Hypothermia depresses ventilatory, renal, and hepatic functions (Moore, 2008). In one large study more than 50% of the trauma patients known to be hypothermic died (Rutherford et al, 1998).
In patients with extensive burns there is a loss of body heat from radiation from the burned tissues and from convection when the tissues are exposed to air currents. Because of the extreme heat loss through the burned tissues, these patients are at high risk for developing perioperative unplanned hypothermia (Corallo et al, 2008; AORN, 2009).
Factors that place patients at an intrinsic risk for developing hypothermia include patient physical status and comorbidities. Endocrine diseases, in particular, cause patients to be more prone to hypothermia (Sessler, 2008). Cardiovascular diseases may cause peripheral vasoconstriction and preemptive hypothermia (Frank, 1995). Thin or small-stature patients with a lack of tissue mass are more likely to become hypothermic. Obese patients generally have low core-to-peripheral temperature gradients and little redistribution hypothermia development. Infants and children cool more quickly because of their high ratio of surface area to weight, which leads to more heat loss through the skin (Kurz et al, 1995). Increased age is considered a predictive risk factor for perioperative unplanned hypothermia. Older adult patients have decreased thermoregulatory efficiency, decreased muscle mass, and changes in vascular tone that inhibit vasoconstriction and decrease heat production (Ayres, 2004).
HYPOTHERMIA AND COMPLICATIONS
Any level of hypothermia can cause serious patient problems. Even mild hypothermia (i.e., temperature less than 36 ° C [96.8 ° F]) has been consistently linked with perioperative complications. Randomized clinical trials have found increased adverse events associated with hypothermia ranging from those that affect basic comfort levels to effects on molecular interactions and cellular functions in a number of systems, including the cardiovascular, integumentary, hematologic, immune, renal, hepatic, and neuroendocrine systems (Wagner, 2006; AORN, 2009). Of course, the more at risk the patient and the more significant the level of hypothermia, the greater the chance of adverse outcomes. A discussion of complications from perioperative hypothermia follows.
Thermal Discomfort
Whether negative or positive in nature, memories of thermal comfort after surgery have an impact on overall patient satisfaction with surgical care. Thermal comfort may have more effects on surgical patient outcomes than nurses formally recognize. Even patients with normal core temperatures may experience thermal discomfort because of a low skin temperature that significantly contributes to thermal sensations. The need for thermal comfort is responsible for the initiation of behavioral thermoregulation (Wagner et al, 1996; Sessler, 2008). Thermal comfort measures may be used throughout all phases of perioperative care; however, during both the intraoperative and immediate postoperative phases of care the patient will need more than a thermal comfort intervention. When a patient is unable to relate in a subjective manner while in a surgical environment, thermal comfort measures become thermal protective or prevention measures during the intraoperative and immediate postoperative phase of care. Thermal protection is an appropriate term or concept that reflects the practice of perioperative nurses especially during the intraoperative and postoperative phases of care. Perioperative nurses frequently use preoperative warmth measures both for prevention of hypothermia and to provide comfort.
In a study examining the relative contribution of core and skin temperatures to thermal comfort and autonomic responses in humans, it was noted that thermal comfort was responsible for the initiation of behavioral thermoregulation and that humans are usually able to control both ambient temperature and the level of body-surface insulation. Findings show skin temperature contributed greatly toward subjective thermal comfort, whereas core temperature predominately regulates the autonomic and metabolic responses (Frank et al, 1999).
Of all the complications of hypothermia reported, shivering is the most frequent and probably the most familiar. It is how the body tries to correct hypothermia and usually occurs in the recovery phase following surgery. Even though shivering is the body’s attempt to generate heat, it actually produces little heat, especially when a patient has received anesthesia. Shivering is metabolically valuable to the patient in that it increases oxygen requirements with the increased metabolic rate and carbon dioxide (CO2) production. Shivering can lead to double or triple the oxygen consumption and carbon dioxide production in postanesthesia patients, although the increases are normally much smaller. Increases in metabolic requirements might predispose patients to further complications if limitations with cardiac or respiratory reserves already exist. Shivering also has been found to increase overall myocardial work and decrease arterial oxygen saturation, mixed venous saturation, and glycogen stores. The stress from shivering frequently results in elevated heart rate, labored breathing, and muscle spasms. Severe shivering may also result in tachycardia, hypertension, and myocardial ischemia, which could lead to cardiac arrest (Sessler, 2008). In addition, shivering increases incisional pain and is often remembered by patients as a fearful experience.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







